Improving Aroma Profiling of Hops by Headspace TD–GC–TOF-MS
The Column
This article describes the use of a headspace thermal desorption–gas chromatography–time-of-flight mass spectrometry (headspace TD–GC–TOF-MS) method to analyze complex aroma profiles from hops, and highlights how it can provide a rapid yet robust approach when comparing similar samples. The article also examines the potential of “soft” electron ionization at 12 eV for distinguishing between structurally similar monoterpenoids and sesquiterpenoids to provide better characterization of the often subtle differences in headspace profiles between different hop varieties.
Photo Credit: imagenavi/Getty Images

This article describes the use of a headspace thermal desorption–gas chromatography–time-of-flight mass spectrometry (headspace TD–GC–TOF-MS) method to analyze complex aroma profiles from hops, and highlights how it can provide a rapid yet robust approach when comparing similar samples. The article also examines the potential of “soft” electron ionization at 12 eV for distinguishing between structurally similar monoterpenoids and sesquiterpenoids to provide better characterization of the often subtle differences in headspace profiles between different hop varieties.
Hops, the female inflorescences of the climbing perennial plant
Humulus
lupulus
, have been used for centuries to impart the distinctive bitter taste and aroma to beer. Many of these bitter and aromatic components originate in the loosely-attached yellow lupulin glands, found on the surface of these hop “cones” (or “strobiles”). The ether- and methanol-soluble portion of hops, known as hop resin, contributes 15–30% of their dry weight, and is primarily responsible for their bitter characteristics. In contrast, the volatile portion (“essential oil”) is responsible for just 0.5–3.0% of the dry weight, but is responsible for providing all the aroma and flavour.
1
Two-dimensional gas chromatography coupled with time-of-flight mass spectrometry (GC×GC–TOF-MS) analysis
2
has indicated that over 1000 compounds may be present in hop essential oil, and to date over 450 components have been identified,3 primarily comprising hydrocarbons (monoterpenes and sesquiterpenes), sulphur compounds (sulphides and thioesters), and oxygenated compounds (alcohols, ketones, and esters).
1
It is worth noting that the vast majority of the compounds in hop essential oil are transformed or lost during conventional brewing processes that involve boiling hops with the liquid “wort”. This is because boiling causes many of the volatile compounds to evaporate, and most of the rest to oxidize or polymerize.1 Adding to the complex picture is the generation of volatile components by the decomposition of hop resin during boiling, and further changes in volatile content during subsequent fermentation. As a result, only a few of the compounds present in hop essential oil make any significant contribution to the aroma of beer – of primary interest are the monoterpene β-myrcene and the sesquiterpenes caryophyllene, β-farnesene, and α-humulene. However, despite these factors, hop volatiles continue to be an active area of research, for two main reasons. The first is that hop aroma profiles are sensitive to a number of factors including hop variety, growing conditions, time of picking, age, and storage conditions.
1
Detailed chemical analysis can identify these differences and is therefore a powerful approach to hop quality control. Secondly, hops are increasingly being added in the later stages of beer brewing (“late hopping” or “dry hopping”), which results in more of the volatile components ending up in the finished beer.
4
The use of hop-derived products (such as liquid-CO
2
extracts) adds an extra dimension of variability. Such approaches offer a route to new products for the beer industry, but naturally place a much greater emphasis on the quality of the hops, which includes minor components as well as major volatiles.
Analysis of Hop Volatiles
The high resolution of GC–MS and its ability to provide accurate and reliable qualitative and quantitative data on chemical composition makes it a natural choice for the analysis of volatile oils of biological origin. TOF-MS offers significant advantages in such scenarios, with instruments using direct extraction (rather than the inherently less efficient orthogonal extraction) providing a distinct sensitivity advantage. However, certain analytes remain challenging even with such technologies, because of their propensity to fragment extensively at conventional (70 eV) ionization energies. This problem can now by addressed by the use of a soft electron ionization (EI) technique that offers the advantages of soft ionization for GC–MS, without any of its historic disadvantages – such as hardware changes, additional reagent gases, and inherent loss of sensitivity. The technique uses an ion source design in the TOF instrument that allows the ionization energy to be varied on a sliding scale from conventional 70 eV to lower energies.
5
The physical properties of most small molecules means that relatively small differences in ionization energies between 10 eV and 20 eV can have significant differences in the fragmentation pattern. Typically, however, an ionization energy of about 12–14 eV retains a useful degree of fragmentation while avoiding a loss in sensitivity relating to the unavoidable drop-off in ionization efficiency as the ionization potential is approached. This technique has already been applied to analytes ranging from petrochemical hydrocarbons
6
to emerging environmental contaminants,
7
and this article shows how this soft ionization method can be applied to hop volatiles, in particular the monoterpenes and sesquiterpenes. These are challenging because extensive fragmentation at 70 eV can make speciation difficult, especially in complex matrices. Using soft ionization provides enhanced “diagnostic” ions and reduced chemical noise, which leads to lower detection limits, as well as simplified spectra for more confident qualification. A further boost in sensitivity is gained by reduced ionization of common background/carrier gases at low ionization energies, leading to minimal chemical noise and improved signal-to-noise ratios for compounds of interest. This article demonstrates how headspace sampling of hops, with collection of vapours onto a sorbent tube and analysis by thermal desorption (TD)–GC–TOF-MS, can be used to generate comprehensive volatile profiles of hops, and how the analysis can be assisted by the use of soft electron ionization.
Experimental
Dynamic Headspace Sampling:
Dried hops (~1 g) were placed in individually sealed and temperature-controlled pots within a Micro-Chamber/Thermal Extractor (Markes International). Volatiles were then extracted under a stream of nitrogen at 150 mL/min for a period of 30 min at 30 °C, and collected onto an inert-coated stainless steel sorbent tube (Markes International) packed with Tenax TA (Markes International). Tubes were then analyzed by TD–GC–TOF-MS using the conditions below.
Conditions:
TD: Dry-purge: 1 min at 50 mL/min. Pre-purge: 1 min at 20 mL/min to split. Tube desorb: 10 min at 280 °C and 50 mL/min trap flow (no split). Focusing trap: Tenax TA. Pre-trap fire purge: 1 min at 50 mL/min. Trap low: 25 °C. Trap high: 280 °C. Trap heating rate: max, hold 5 min. Split flow: 150 mL/min (126:1), collected onto a clean Tenax TA sorbent tube.
GC:
Column: 30 m × 0.25 mm, 0.25-μm (Agilent Technologies). Carrier gas: Helium, 1.2 mL/min. Oven temp: 40 °C for 5 min, then 4 °C/min to 280 °C (hold 5 min). Total run time: 70 min.
TOF-MS:
Data rate: 5 Hz. Mass range:
m/z
35–500. Ion source: 230 °C. Transfer line: 280 °C. Filament voltage: 1.8 V.
Software:
TOF-DS software (Markes International) was used for instrument control, background-subtraction, deconvolution, integration, and library searching. All samples were screened against the NIST 14 library as acquisition proceeded, and identifications were confirmed on the basis of a match factor >750.
Results and Discussion
Analytical
Approach: Sampling used a dynamic headspace technique, which avoids the need for sample preparation, and has previously been used successfully to aroma-profile strawberries.
8
Three general-purpose hop varieties used in English ales and lagers were studied – “Fuggle”, “Goldings”, and “Target”. Use of a slightly raised temperature (30 °C) allowed the efficient release of a representative sample of volatiles within a reasonable time-frame, without risking the loss of the more volatile species from the sorbent tube, as shown by previous work.
9
An ambient-temperature dry-purge of the sampled sorbent tube was used to remove excess moisture from the sample before thermal desorption and secondary focusing of the volatiles on a cold trap. Subsequent rapid heating of the cold trap was performed with an outlet (trap desorption) split of 126:1, to ensure that the detector was not saturated by the high-abundance components in the relatively concentrated vapour samples.
Varietal Comparison:
The aroma profiles of the three hop varieties are shown in Figure 1, with the profiles showing a division of analytes into two broad regions corresponding to the monoterpenoids (all eluting before 19 min), and the sesquiterpenoids (eluting after 26 min).
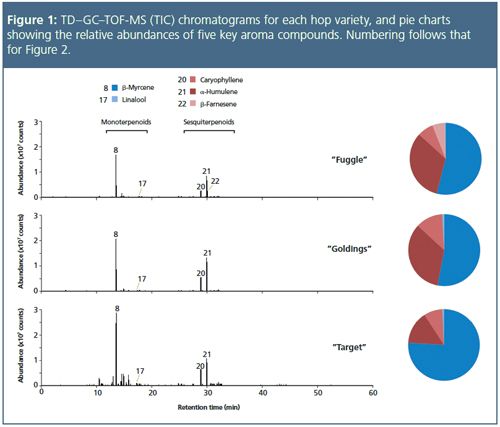
The expansions shown in Figure 2 indicate some of the minor components identified – attributed aromas are indicated. β-Myrcene (#8 – musty, sweet, lemon, spicy, woody
10
), caryophyllene (#20 – oily, fruity, woody
10
), and α-humulene (#21 – musty, spicy, woody
10
) dominated in all three samples, albeit with different relative abundances. Interestingly, with the exception of β-farnesene (#22 – oily, fruity, citrus-like, woody
10
) in “Fuggle”, the three varieties show remarkably similar profiles at higher retention times, reflecting a similar balance of less volatile components.
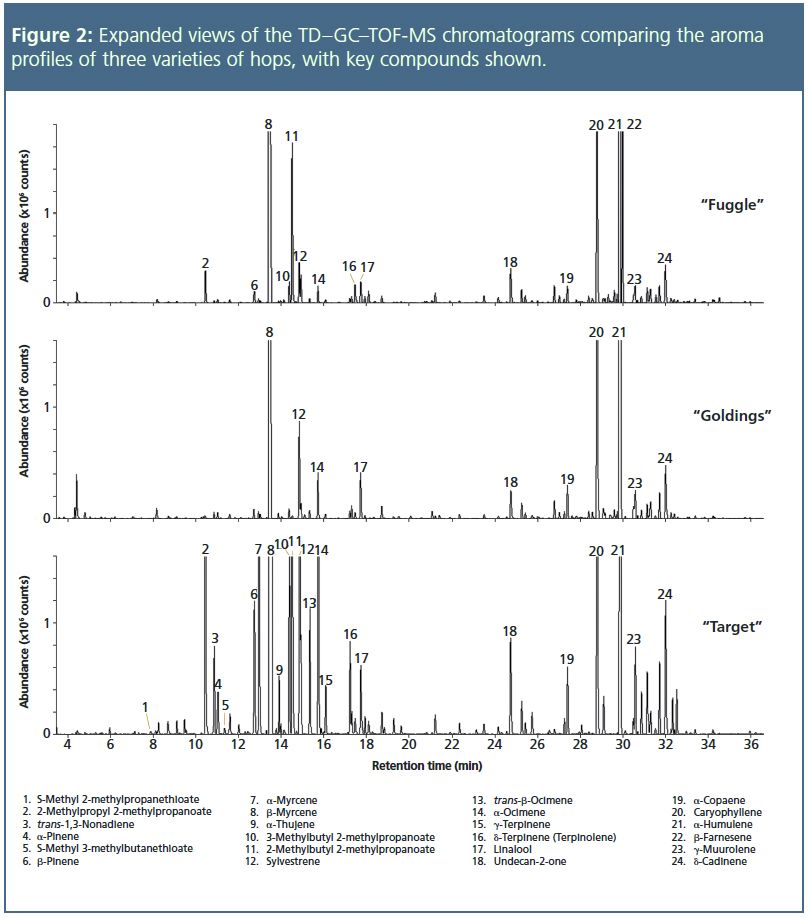
“Fuggle” and “Goldings”, both traditional English medium-strength floral/aromatic varieties, have very similar overall profiles, with the only significant differences being the presence in “Fuggle” of 2-methylpropyl 2-methylpropanoate (#2 – pineapple
11
) and 2-methylbutyl 2-methylpropanoate (#11 – fruity, green, apple-like, apricot
12
), in addition to β-farnesene (noted above). “Target”, on the other hand, shows considerable differences from “Fuggle” and “Goldings” that reflect its more robust aroma. These include the presence (in significant quantities) of β-pinene (#6 – musty, green, sweet, pine
10
), α-myrcene (#7), 3-methylbutyl 2-methylpropanoate (#10 – fruity, pineapple, apricot
11
), trans-β-ocimene (#13 – green, tropical, woody, floral
11
), and δ-terpinene (#16 – sweet, pine, citrus
11
). Undecan-2-one (#18 – fruity, musty, dusty, green
10
, citrus
13
) is also found in higher abundance in “Target”, as previously noted by Lermusieau and Collin
14
, who were able to differentiate this variety from other European hops by a high abundance of this compound. Two sulphur-containing compounds, S-methyl 2-methylpropanethioate (#1) and S-methyl 3-methylbutanethioate (#5), were both also only found in “Target” – both have been reported to contribute a “cheesey, estery, cooked vegetable” aroma,
15,16
and for this reason may be undesirable.
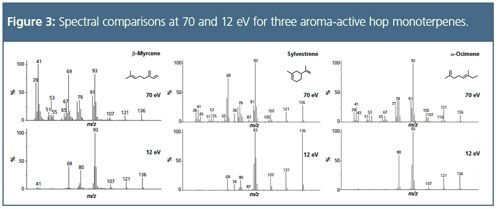
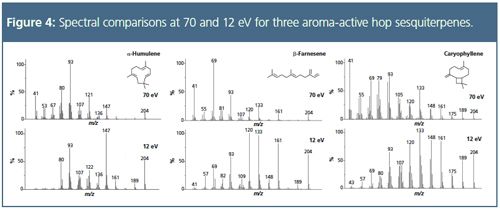
Improving Isomer Speciation:
Even with careful choice of sampling and chromatographic parameters, and the use of TOF-MS to provide high sensitivity across a wide mass range, analysis of samples containing terpenes and sesquiterpenes presents difficulties when conventional 70 eV ionization energies are used. This is because of their weak molecular ions, lack of distinctive fragmentation patterns (because of the absence of heteroatom functional groups), and the spectral similarity of compounds that differ only in the position of a carbon–carbon double-bond or methyl group. To address this, the split flows from TD analysis of each sample were re-collected onto clean sorbent tubes, and the analysis repeated using soft ionization at 12 eV. Spectral comparisons for monoterpenes and sesquiterpenes are shown in Figures 3 and 4, respectively. Note that this approach to soft ionization, although providing an increased intensity for the molecular ion, does not completely eliminate fragmentation. This limited degree of fragmentation provides improved intensity for the high-molecular-weight ions that are of greatest value diagnostically, resulting in increased ability to distinguish confidently between similar components. This approach to soft ionization offers a particular advantage for the brewing industry, because although aroma compounds are present in concentrated form in hop essential oil and hop headspace, the concentrations of these compounds are much lower in the final brewed beer, and may be masked by the large number of compounds that arise during the brewing process. A proof-of-concept analysis such as that illustrated here, tested on hop volatiles, could be extended to beer, with the bulk of the components identified at 70 eV, and a follow-up run from an identical re-collected sample at 12–14 eV to confirm doubtfully-identified analytes, especially those at trace levels or masked by co-eluting compounds. In relation to this, a database of low-eV electron ionization spectra is currrently being developed,
17
which should assist analysts investigating samples using this approach.
Conclusion
This article demonstrates that TD–GC–TOF-MS can be successfully applied to the analysis of strongly aromatic plant materials such as hops, with dynamic headspace sampling onto sorbent tubes allowing the collection of representative profiles from multiple samples in a short time-frame. The use of time-of-flight mass spectrometry provides information-rich datasets, while soft electron ionization adds a new dimension to the data available from MS, while maintaining high sensitivity. Together, these capabilities mean that this approach enables robust comparison between samples, with greater confidence in compound identity. It should consequently be valuable for quality control of hops and beer in the brewing industry.
References
[1] C. Almaguer et al., J. Inst. Brew.120, 289–314 (2014). [2] M.T. Roberts et al., J. Sep. Sci. 27, 473–478 (2004). [3] L.M. Nijssen et al., Volatile Compounds in Food (VCF): Database (2010). Available at: http://www.vcf online.nl/VcfHome.cfm. [4] C. Schönberger and T. Kostelecky, J. Inst. Brew. 117, 259–267 (2011). [5] L. McGregor et al.,, LCGC Supplement: Current Trends in Mass Spectrometry 12(1), 16–19 (2014). [6] L. McGregor and D. Barden, Analyzing crude oil: Improving compound speciation, Hydrocarbon Engineering (2014) http://www.energyglobal.com/downstream/gas-processing/31072014/Crude-oil-markes analysis/ [7] L. McGregor et al., The Analytical Scientist, April 2015. [8] P. Morris and D. Barden, The Column 9(8), 9–13 (2013). [9] T. Schripp et al., Anal. Bioanal. Chem. 387, 1907–1919 (2007). [10] J.L. Gonçalves et al., Food Chem.160, 266–280 (2014). [11] G.A. Burdock, Fenaroli’s Handbook of Flavor Ingredients (5th edition, CRC Press, 2004) [12] K. Takoi et al., “Identification of novel unique flavor compounds derived from Nelson Sauvin hop and development of a new product using this hop,” paper presented at the 31st European Brewery Convention Congress, 2007 https://www.researchgate.net/publication/261476629_Identification_of_novel_unique_flavor_compounds_ derived_from_Nelson_Sauvin_hop_and_development_of_a_new_product_using_this_hop [13] F. Van Opstaele et al., J. Agric. Food Chem. 61,10555−10564 (2013). [14] G. Lermusieau and S. Collin, in Analysis of Taste and Aroma (Molecular Methods of Plant Analysis) (vol. 21), J.F. Jackson and H.F. Linskens, Eds. (Springer, 2002) pp. 69–88. [15] T.L. Peppard, J. Inst. Brew. 87, 376–385 (1981). [16] G. Lermusieau and S. Collin, J. Am. Soc. Brew. Chem. 61, 109–113 (2003). [17] Select-eV library, Markes International. Available at: http://www.markes.com/select-ev-library/.
Acknowledgements
Markes International gratefully acknowledges the Barth Haas Group for providing the hop samples examined in this study.
Stefan
Koschinski
is a state-approved food chemist and received his degree in food chemistry from the Goethe University of Frankfurt. He carried out his Ph.D. studies at the Dienstleistungszentrum Ländlicher Raum (DLR) Rheinpfalz (Germany) and received his Doctor of Natural Sciences from Kaiserslautern University of Technology (Kaiserslautern, Germany). His studies were focused on the influence of terroir on the aroma of Riesling wines using several analytical techniques, including GC–MS and GC×GC–MS. Stefan joined Markes in 2012 as a technical specialist for Markes’ TD and TOF product portfolio in the DACH region.
Laura McGregor
received an M.Chem. in chemistry from the University of St Andrews, UK, followed by an M.Sc. in forensic science at the University of Strathclyde, UK. Her Ph.D. in environmental forensics, also at the University of Strathclyde, focused on the chemical fingerprinting of environmental contamination using advanced techniques such as GC×GC−TOF-MS. Laura joined Markes International in 2013 as a sales support specialist, and is now product marketing manager for Markes’ TOF-MS product portfolio.
Gareth Roberts
has worked in the field of GC and GC–MS since 1980, after graduating with a first-class honours degree in chemistry. After a period of research at the Shell Thornton establishment, he worked at PerkinElmer, followed by 20 years at Hewlett Packard/Agilent Technologies. Gareth joined Markes in 2003 as an international business manager, focusing on thermal desorption and GC–TOF-MS, in various applications associated with the defence, forensic, fragrance, and food industries.
David Barden
is a technical copywriter at Markes International, having joined the company in 2011. David studied natural sciences at the University of Cambridge, UK, and remained there for his Ph.D. in organic chemistry, which he received in 2003. A placement at the European Journals Department of Wiley-VCH, Weinheim, Germany, was then followed by seven years in journal publishing at the Royal Society of Chemistry, UK.

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.













