Improving the Analytical Workflow for Protein Biopharmaceutical Characterization with a Novel LC–MS System Solution
Special Issues
The ability to characterize protein therapeutics throughout the product development cycle is discussed.
Nearly every process conducted in a biotechnology company requires a tremendous amount of analytical characterization in support of the development of biopharmaceutical products. Because of the inherent complexity of biotherapeutics, characterization of their large biomolecules is significantly more difficult than it is for small molecules. Therefore, numerous instrumental methods are applied to fully characterize these high molecular weight products for regulatory approval, intellectual property protection, or comparability studies during manufacturing changes.
The key to the full characterization of complex biopharmaceutical samples lies in the use of appropriate combinations of the differing available methods to analyze the sample using independent physicochemical properties. The involvement of many analytical methods in the characterization process calls for an integrated workflow — a process through which information regarding the biomolecules can be acquired both reliably and seamlessly. Such a workflow not only includes high-performance instrumentation but also integrates powerful bioinformatics tools so that comprehensive characterization data can be obtained in a routine manner without compromises.
Liquid chromatography–mass spectrometry (LC–MS) plays an important role in the ensemble of analytical tools for in-depth characterization of biotherapeutic products because it reveals the structure and stability of biopharmaceuticals. Information acquired by an LC–MS approach is used to demonstrate product quality and consistency, and to identify the desired or undesired forms of protein products. Structural analysis is particularly important to the production process as small changes in an optimized process can affect the structure of the product. And, as every chemist knows, ensuring sufficient pharmaceutical stability is a great challenge in protein drug formulation development because a large number of functional groups in protein therapeutics are susceptible to chemical degradation.
This article focuses on two distinct LC–MS system solutions — for intact mass analysis and peptide mapping — that can be utilized for product confirmation or for the detection of minute changes that are associated with a humanized monoclonal antibody (mAb). For each solution, an integrated workflow is presented.
This workflow features the resolution, sensitivity, and speed of ultrahigh-pressure liquid chromatography (UHPLC) separations, application-specific column chemistries, sensitive and accurate-mass MS, MSE , and MS-MS detection, and biopharmaceutical informatics. Used together in this workflow, they deliver unparalleled improvements in laboratory productivity.
In each of the following sections, the humanized monoclonal antibody is analyzed to demonstrate how scientists can obtain high-quality information routinely, without the need for advanced expertise.
Intact Protein Mass Analysis and a Case Study for Identifying the Difference Among Production Batches of a Monoclonal Antibody
Coupling LC with MS has given biopharmaceutical scientists detailed, accurate, and interpretable information. For example, one rapid LC–MS experiment for intact mass analysis can help to determine if the biomolecule has been expressed correctly and post-translationally modified. It also provides an overall view of the heterogeneity of the protein and relative amounts of the various glycosylated forms.
Intact mass analysis is used for comparing the glycoprofiles of different mAbs expressed in similar cell lines and under comparable conditions. Knowing the distribution of glycoforms of protein drugs helps to save significant product development time and cost. For a finished drug product, intact mass analysis represents a rapid and convenient method that quickly confirms identity. Demonstrating the comparability of proteins produced throughout product manufacturing is critical to obtain regulatory approval for scale-up to production.
The value of intact mass analysis during the protein development process largely depends upon the efficiency of an intact protein analysis approach:
- The chromatography system should be able to resolve a complex protein mixture if needed. On the other hand, if quick mass measurement for a pure protein sample is required, the LC system needs to efficiently remove MS-interfering species from a variety of samples and sample matrixes.
- MS employed for intact mass analysis should be easy to use and have good sensitivity and wide mass range such that proteins can be measured accurately, large or small.
- More importantly, because intact protein mass spectra typically contain a charge envelope, researchers need a highly efficient informatics tool that automatically and accurately converts the protein raw spectra into the zero-charged protein mass and provides abundance-related information for various protein isoforms.
As common as the practice of intact protein mass analysis is, there is still a great need for an effective workflow that offers high-performance LC–MS data collection along with subsequent data processing so the intact mass measurements can be executed readily, and high-quality information for drug development can be obtained routinely.
Until recently, intact mass analysis and data interpretation was largely the domain of LC–MS experts. There were not many usable tools available for scientists to obtain data without considerable experience and know-how — and even fewer that converted raw data into usable information.
In the examples discussed here, intact mass analysis was performed on a humanized monoclonal antibody (IgG1) using this workflow. The objective of the analysis is to assess the variability of glycoforms for a multiple IgG-based therapeutic using a rapid on-line desalting method. Detailed experimental parameters are listed in Table I.
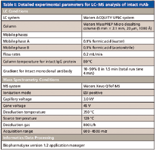
Table I: Detailed experimental parameters for LCâMS analysis of intact mAb
Experimental Method and Results for Intact Mass Analysis
Samples (IgG1)
A humanized monoclonal antibody was received as a buffered solution (21.0 mg/mL). The solution was diluted to 0.5 mg/mL with 50 mM ammonium bicarbonate in preparation for intact mass analysis. The conditions for this experiment are shown in Table I.
When proteins as large as a monoclonal antibody are subjected to electrospray ionization, multiply charged ions are formed. Figure 1a shows the charge state distribution of the IgG1 from one production batch. The charge envelope is centered at the 50+ charge state. When the spectrum is examined in greater detail, as in the inset of Figure 1a, where the 47+ charge state is shown, it can be observed that each charge state consists of multiple peaks. After this spectrum is deconvoluted by MaxEnt1 (Figure 1b), the glycosylation heterogeneity of the IgG1 is displayed clearly. This deconvoluted spectrum forms the basis on which the glycoform variations of different production batches are compared.
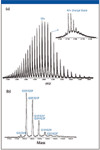
Figure 1: (a) Combined ESI-QTof mass spectrum of an intact IgG1 for the 0.5-μg load. A charge state envelope of 2100â4100 m/z was observed for the intact IgG1, centered on 50+ charge state of the protein. The 47+ charge state region has been enlarged to show detail and reveals at least six major antibody variants even before MaxEnt1 processing. (b) MaxEnt1 deconvoluted mass spectrum of the intact IgG1. Major variants observed were due to carbohydrate heterogeneity.
Figure 2 displays the mass spectra of the same intact IgG1 analyzed by a traditional quadrupole time-of-flight (Q-TOF) mass spectrometer and a Xevo QTof MS system (Waters Corporation, Milford, Massachusetts). Both spectra show a charge envelope with a distribution of multiply charged ion peaks. Although great similarity between the two spectra can be found, the Xevo QTof MS data demonstrate a sixfold increase in sensitivity compared with the traditional Q-TOF system.
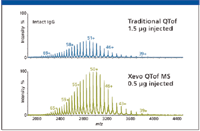
Figure 2: For the intact analysis, the Xevo QTof MS shows a sixfold improvement in sensitivity in comparison to tradition quadrupole time-of-flight MS.
The intact mass analysis solution is applied next to assess variability of glycoforms of the therapeutic from multiple production batches. Batch comparisons of multiply produced IgG require a substantial number of samples to be examined quickly. The method must also provide a meaningful way to demonstrate the comparability of produced proteins within a short timeframe.
The online desalting LC–MS method (1) coupled with BiopharmaLynx software (Waters Corporation) is well suited for such a task. MS data were collected on the Xevo QTof MS system with a 4-min run time. Spectral deconvolution for the large dataset is performed automatically by the software using the embedded MaxEnt1 algorithm. Typically, for a dataset with 125 injections (8 h of data collection time), it takes less than 30 min for the software to process all the data and generate meaningful information. In contrast to manual deconvolution processes, parameter settings in the software are handled automatically by the software, thus making the parameters better-fitted to each individual data file. In addition, a large dataset can be handled under one common set of processing parameters to avoid any human bias.
Figure 3a is a mirror plot showing the comparison of the deconvoluted spectra of the IgG1 from the same production batch. The software features an interactive browser page that allows a pair of datasets (chromatograms or mass spectra) to be plotted in stacked or mirror format for visual examination. Furthermore, the processed and tabulated data contain pertinent information about intact proteins, such as the abundance of various glycoforms.
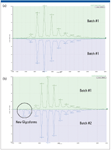
Figure 3: (a) MaxEnt1 deconvoluted mass spectra of the antibody from two injections of the same production batch, and (b) MaxEnt1 deconvoluted mass spectra of the antibody from two dissimilar production batches. Two new minor glycoforms are observed in batch 2.
Using the comparison metrics, a number of samples can be rapidly compared to a well-studied sample (control) and reports are generated automatically for external users. As expected, Figure 3a does not show any glycoform variations as both datasets were generated by the IgG from the same production batch. In comparison, Figure 3b shows the deconvoluted spectra for the IgG1 from two different production batches, where the difference between some minor glycoforms of the IgG1 is clearly observed and labeled.
LC–MS Peptide Mapping and Detection of Minute Changes in a Monoclonal Antibody
Peptide mapping is the workhorse technique in biopharmaceutical analysis, offering the comprehensive characterization of biopharmaceutical products. Its applications include the identification of proteins based upon the elution pattern of peptide fragments, the determination of posttranslational modifications, the confirmation of genetic stability, and the analysis of protein sequences when interfaced to a mass spectrometer.
In a typical peptide mapping workflow, the protein is first digested with trypsin or other protease to generate peptides, which are separated by liquid chromatography, most commonly reversed-phase LC, and then detected by either UV–vis or MS. Compared with a traditional LC–UV-vis approach, LC–MS analysis significantly enhances the information content available from a peptide mapping experiment. Measurement by MS can differentiate coeluted peptides under a chromatographic peak and can identify and locate low-abundance modifications and single amino acid variants of protein by MS-MS.
Although LC–MS peptide mapping can be powerful in detecting and identifying minute changes in a protein therapeutic, many organizations struggle to develop a robust and reproducible analytical workflow. Poor sample preparation, faulty chromatographic methodology, low resolution, inaccurate measurement, and time-consuming manual data annotation all can diminish the utility of this technique.
In this section, the effectiveness of the workflow is demonstrated through an example that identifies and quantifies N-deamidation progression in a humanized monoclonal antibody during a forced degradation study.
Experimental Method and Results for Peptide Mapping
Sample Preparation
RapiGest SF surfactant (Waters Corporation) (0.05% in final solution) was added to the monoclonal antibody stock solution, and the sample was heated at 60 °C for 30 min for protein denature. The protein was then reduced with 10 mM DTT at 60 °C for 30 min and alkylated with 13 mM of IAA in the dark for 45 min. Trypsin digestion was performed at 37 °C for 4 h (trypsin:protein = 1:50). The pH of the resulting digest was adjusted to ~ 9 by adding 1 M ammonium hydroxide. Then, the digest was aliquoted into five portions and incubated at 60 °C for 0, 5, 10, 30, and 60 min, respectively. Each portion was diluted to 1.5 pmol/μL with 95:5 water–acetonitrile containing 0.1% formic acid before LC–MSE analysis. The conditions for this experiment are shown in Table II.
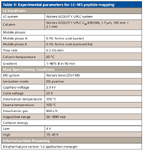
Table II: Experimental parameters for LCâMS peptide mapping
To obtain a reproducible peptide map, protein digestion should be highly consistent and reproducible for the selected protein over the course of study. To ensure the most consistent digestion, RapiGest SF surfactant is included in the digestion protocol. The surfactant used here is an MS-friendly surfactant that denatures the protein while causing no harm to enzymatic activity of many proteases. By using this surfactant in the digestion protocol, the inclusion of a common denaturant such as urea and guanidine hydrochloride in the digestion buffer is avoided. These latter denaturants are known to potentially cause peptide modification or inhibit trypsin activity during the digestion. The advantages of of this surfactant in protein digestion are well documented in the literature (2). With this surfactant, the protein was completely digested within 4 h, which minimizes artificial deamidation that could be produced during typical digestion processing (3).
To make a peptide map, the goal is to separate every peptide into a single peak. Because of the complexity of peptide digests, peptide mapping represents a major chromatographic challenge. Typically peptide mapping involves shallow gradients with long run times. UHPLC is known to increase resolution, speed, and sensitivity in a variety of applications. When applying UHPLC to peptide mapping, UHPLC peptide maps show higher resolution than other methods and increased sensitivity (4). In addition, UHPLC generates particularly good peak shapes for glycopeptides and demonstrates good resolution for deamidated peptides (5).
The capabilities of UHPLC in a higher-resolution peptide mapping experiment are exemplified in Figure 4, in which a typical LC–MS peptide map for a mAb is shown. On average, the peak width of the peptides is on the order of 9 s (half height) for the UHPLC conditions listed in the experimental section.
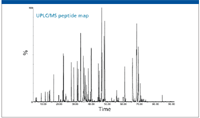
Figure 4: Total ion chromatogram of the tryptic digest of the monoclonal antibody. The data were collected from an ACQUITY UPLC/Xevo QTof MS system (Waters Corporation) with 10 pmol of protein digest injected.
Peptide mapping with MS detection is a valuable means for achieving detailed and comprehensive modification coverage in a simple manner. Although single-stage LC–MS can provide considerable information regarding peptide identity, simple mass measurement cannot differentiate isobaric peptides. Furthermore, it is extremely challenging to identify and localize peptide modifications from MS data alone, especially when the modifications only lead to small or no mass changes.
For these reasons, MS-MS techniques often are used to generate fragment ion mass spectra for detailed sequence analysis and for the location of peptide modifications. Confidence in the information acquired also is enhanced by using instrumentation that provides accurate mass data due to the corresponding significant reduction in ambiguity. Thus a high-resolution, high-mass-accuracy tandem mass spectrometer offers great advantages in peptide mapping.
MS–MS data traditionally are obtained with selected peptides using data-dependent (or directed) acquisition (DDA). In the DDA approach, peptides are selected for fragmentation based upon the intensity of precursor ions. Ions with higher intensity have a greater chance to be selected for fragmentation. Because many of the peptide changes (modifications) in a therapeutic protein occur at a relatively low level, there is a high probability that these interested peptides may not be selected and fragmented during the run.
A significant improvement over the DDA method is to use the multiplexed parallel precursor fragmentation approach (MSE) (6,7) in which all the peptides eluted off the UHPLC column are fragmented without any precursor selection. MSE provides a comprehensive MS-MS picture with no prior knowledge of the sample and is applicable across all charge states (8–11). This means that the peptide map can be performed in an unbiased, systematic manner and will provide consistent results each time. The technique also means that the same historical dataset could potentially be reanalyzed at a later stage for additional information with no need to rerun the samples, saving significant laboratory costs, time, and capital investment.
Figure 5a shows the processed (deisotoped, charge-reduced, and centered) MS spectra of a tryptic peptide (HT36, 1161.62 Da) and the deamidated counterparts (1162.61 Da) from the forced degradation study of the mAb digest. The top panel is from the untreated sample and the bottom panel is from the sample after 60-min incubation at pH 9.0 and 60 °C. The relative intensity ratio of the two peptides is about 500 in the untreated sample.

Figure 5: (a) Identification of peptide HT36 and N364-deamidated HT36, by accurate masses processed by BiopharmaLynx 1.2. The mirror plot shows a comparison between samples at 0 and 60 min incubation time. (b) MSE confirmation of N364-deamidation in HT36 by comparing MSE spectra at RT 44.7 and 47.9 min, processed by BiopharmaLynx 1.2 (note the difference of y10 and b-series ions).
The software's graphical view quickly offers three pieces of information: mass assignment of HT36 and the deamidated HT36; relative amount of the deamidated HT36 in each digest; and abundance difference of N-deamidation between the two digests. More importantly, the identification of deamidation of HT36 and its deamidated form were further confirmed by the MS-MS data acquired by MSE. Because no precursor selection is involved during the run, MS-MS data for both HT36 and its deamidated form were acquired simultaneously, despite the great difference in ion intensities. Figure 5b displays the comparison of the MS-MS spectra for the HT36 peptide and one of the deamidated isoforms. A 1-Da mass difference of b-series ions in the MSE spectra, also processed by the software, easily confirms that the deamidation takes place at the N-terminal asparagine residue.
Data processing and annotation frequently becomes one of the bottlenecks in the peptide mapping workflow. Although the benefits of MS for peptide-mapping studies are greatly appreciated, MS ionization also adds enormous complexity to data interpretation and annotations. For instance, peptides tend to ionize in multiple charge states; different peptides at the same concentration may show tremendous variation in MS responses, and peptides may experience in-source fragmentation. To completely annotate each of the ions observed in multiple LC–MS runs by manual processing is a time-consuming task.
Figure 6 shows extracted ion chromatograms of peptide HT36 as well as the deamidated forms. The unmodified peptide is eluted at 44.7 min, and the deamidated peptides are identified at the retention times of 46.7 and 47.9 min, which represents two isoforms of N-deamidation — aspartic acid and isoaspartic acid. An additional peak with 1 Da mass difference was also observed at retention time of 47.0 min, which was identified as glutamine (Q) deamidation by the MSE data.
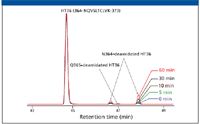
Figure 6: Extracted ion chromatograms of peptide T36 and N364-deamidated T36 of the antibody heavy chain for samples from 0 to 60 min incubation time.
The degree of modifications at each site, including unmodified HT36, can be tracked easily using a Cross Analyte Comparison table in the software for multiple data point comparison across the time course study. For example, the Cross Analyte Comparison table for N-deamidated HT36 eluted at ~46.7 min is shown in Figure 7. These tables provide results for direct monitoring of the N-deamidation progression in the digests. This identification and quantification is consistent with the manual processing results obtained from extracted ion chromatograms of tryptic peptide HT36 and deamidated HT36 in the digests incubated from 0 to 60 min (Figure 7). As expected, the percentage of N-deamidated HT36 increases consecutively, while unmodified HT36 decreases with longer incubation time.
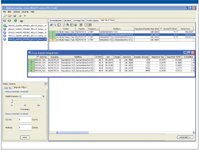
Figure 7: Progression of deamidation in peptide HT36 for samples from 0 to 60 min incubation time, monitored by BiopharmaLynx.
Using a forced degradation study, this work presents an integrated workflow for the LC–MS peptide mapping experiment. The workflow covers a complete system solution targeted for high-performance routine analysis of peptide mapping. As shown, this peptide mapping technique readily generates comprehensive information about the studied sample.
Conclusions
A true laboratory workhorse system must demonstrate the capacity for routine usage across a variety of applications. In this article, we have illustrated how high-performance instrumentation, chemistry, and bioinformatics can be integrated together to form a total system solution for routine characterization of intact proteins and peptide maps, without the need to rely upon the expertise of any single scientist to perform MS. The attributes of these techniques translate into increased user confidence in results that are achieved faster than possible with traditional methods. The solutions are simple and straightforward, and the results are coherent and comprehensive. The ability to quickly, routinely, and comprehensively characterize biopharmaceutical products will improve and accelerate the drug development process, ensure the quality of biopharmaceuticals, and protect intellectual property.
Asish B. Chakraborty, Hongwei Xie, St John Skilton, John C. Gebler, and Weibin Chen are with Biopharmaceutical Sciences, Waters Corporation, Milford, Massachusetts.
References
(1) Rapid Profiling of Monoclonal Intact Antibodies by LC/ESI-TOF MS. Waters Corporation. 2007; 720002393en.
(2) Y.Q. Yu, M. Gilar, P.J. Lee, E.S. Bouvier, and J.C. Gebler, Anal. Chem. 75(21), 6023–6028 (2003).
(3) X. Li, J.J. Cournoyer, C. Lin, and P.B. O'Connor, J. Am. Soc. Mass Spectrom. 19(6), 855–864 (2008). Epub 2008 Mar 5.
(4) J. Mazzeo, T. Wheat, B. Gillece-Castro, and Z. Lu, BioPharm International, 19(1), 22–34 (2006).
(5) UPLC Technology for the Analysis of Antibody Glycopeptides. Waters Corporation. 2007; 720002382en.
(6) S.J. Geromanos, J.P. Vissers, J.C. Silva, C.A. Dorschel, G.Z. Li, M.V. Gorenstein, R.H. Bateman, and J.I. Langridge, Proteomics 9(6), 1683–1695 (2009).
(7) A.B. Chakraborty, S.J. Berger, and J.C. Gebler, Rapid Commun. Mass Spectrom. 21(5), 730–744 (2007).
(8) R.H. Bateman, R. Carruthers, J.B. Hoyes, C. Jones, J.I. Langridge, A. Millar, and J.P. Vissers, J. Am. Soc. Mass Spectrom. 13(7), 792–803 (2002).
(9) J.C. Silva, R. Denny, C.A. Dorschel, M. Gorenstein, I.J. Kass, G.Z. Li, T. McKenna, M.J. Nold, K. Richardson, P. Young, and S. Geromanos, Anal. Chem. 77(7), 2187–2200 (2005).
(10) J.C. Silva, M.V. Gorenstein, G.Z. Li, J.P. Vissers, and S.J. Geromanos, Mol. Cell. Proteomics 5(4), 589–607 (2006).
(11) C.A. Srebalus Barnes and A. Lim, Mass Spectrom. Rev. 26(3), 370–388 (2007).

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Quantifying Terpenes in Hydrodistilled Cannabis sativa Essential Oil with GC-MS
April 21st 2025A recent study conducted at the University of Georgia, (Athens, Georgia) presented a validated method for quantifying 18 terpenes in Cannabis sativa essential oil, extracted via hydrodistillation. The method, utilizing gas chromatography–mass spectrometry (GC–MS) with selected ion monitoring (SIM), includes using internal standards (n-tridecane and octadecane) for accurate analysis, with key validation parameters—such as specificity, accuracy, precision, and detection limits—thoroughly assessed. LCGC International spoke to Noelle Joy of the University of Georgia, corresponding author of this paper discussing the method, about its creation and benefits it offers the analytical community.











