Implementing Pharmacopeial Methods —Method in the Madness?
Incognito leads us through the complex method used to determine related substances for hydroxychloroquine, exploring the practical implementation and critical evaluation of a less than optimum ultrahigh-pressure liquid chromatography (UHPLC) pharmacopeial method.
I’ve recently been involved in troubleshooting some issues with a Pharmacopeial method which have highlighted some important general issues in the practical implementation of high performance liquid chromatography (HPLC) and ultrahigh-pressure liquid chromatography (UHPLC) methods.
The method is used to determine Related Substances for Hydroxychloroquine and is briefly summarized below.
Sample diluent: Dilute sulphuric acid (approximately pH 2.8) : Methanol 50:50
Buffer solution: Water containing potassium dihydrogen phosphate, sodium heptane sulphonate adjusted to pH 7.0 with triethylamine
Test solution (1): 25 mg of test substance diluted to 50 mL with sample diluent
Test solution (2): Test Solution 1 diluted 1:10 (v/v) with sample diluent
Reference Solutions: Various dilutions of Hydroxychloroquine sulphate with known impurities B and C
Column: End-capped ethylene-bridged octadecylsilyl silica gel for chromatography (hybrid material) R (1.7 μm)
Temperature: 40 °C
Mobile phase A: Methanol R, buffer solution (10:90 v/v)
Mobile phase B: Buffer solution, methanol R (15:85 v/v)
Gradient: (See Table 1)
Flow rate: 0.7 mL/min
Detection: Spectrophotometer at 254 nm
Injection: 4 µL of test solution (1) and reference solutions.
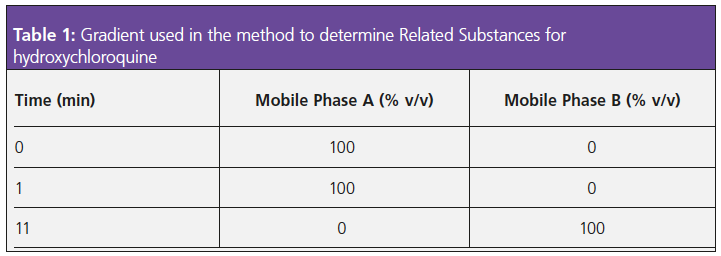
For reference, Figure 1 shows the structures and some physicochemical information for hydroxychloroquine and impurities B and C for which limits are specifically set within the method.
It should be noted that in total there are seven named impurities for this related substance determination, but only those which have individual limits are shown for illustrative purposes.
The issues highlighted by my correspondent included:
- System over-pressure
- Non-reproducible quantitative response (peak areas) for specific analytes
- Retention time drift (often severe)
- Carry-over of the active pharmaceutical ingredient and inability to obtain a “blank” chromatogram despite flushing the system with various reagents
- Noisy and erratic baselines, especially after filtration of the mobile phase.
You may be asking yourself why this very specific set of problems with a very particular Pharmacopeial method warrant Incognito column inches. Simply put, this method, and the problems which have been encountered, highlight some very useful considerations when undertaking liquid chromatographic analysis and the skill of “critical evaluation” of methods, that is, the evaluation of a method to help understand the possible and actual problems encountered. So, whether you work in the pharmaceutical or any other industry, the following guidance should be generally applicable.
The Mobile Phase
Let’s start by looking at the mobile phase and its constituents.
For me, with over 30 years experience in practising chromatography, the constituents of the mobile phase are recognisable but certainly not what one could call “modern”. However, for some less experienced chromatographers, the mobile phase additives, and the fact that the pH is titrated to a set value using one of the reagents will be, frankly, alien.
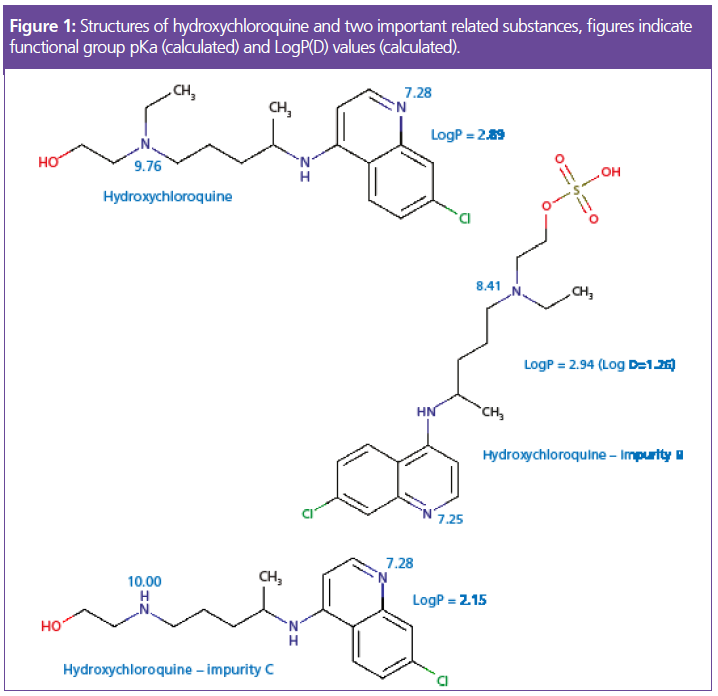
Figure 2 shows the structures of the additives used in the mobile phase and it’s worth spending a little time on why these reagents may be used.
Potassium dihydrogen phosphate is a traditional buffer which is used to resist small changes in pH when the sample in its diluent (approximately pH 2.8) mixes with the eluent within the instrument tubing and primarily at the head of the analytical column. As we wish to maintain a pH of 7.0 to enable reproducible separation, and because the pKa of the phosphate buffer is 7.21, this obeys the general rule that the buffer will be most effective when the desired solution pH is within 1 pH unit of its native pKa. This should lead to an improvement in the robustness of retention time and peak shape. We will further discuss this particular reagent later.
Sodium heptane sulphonate is a so‑called ion pairing reagent, which has two modes of action. In the bulk mobile phase, the anionic reagent will form an ionic equilibrium with basic analyte functional moieties to form a neutral complex, which will be more highly retained on the highly hydrophobic stationary phase surface. As can be seen from the analyte pKa values in Figure 1, at an eluent pH of 7.0, several of the analytes will have protonated functional groups and therefore will be capable of ion pair formation. The second mode of action of the ion pair reagent is to strongly partition into the stationary phase via the highly hydrophobic moiety of the pairing reagent which will effectively add anionic groups (SO3-) to the stationary phase surface, with which any unpaired cationic analyte species may undergo electrostatic attraction, and therefore improve retention.
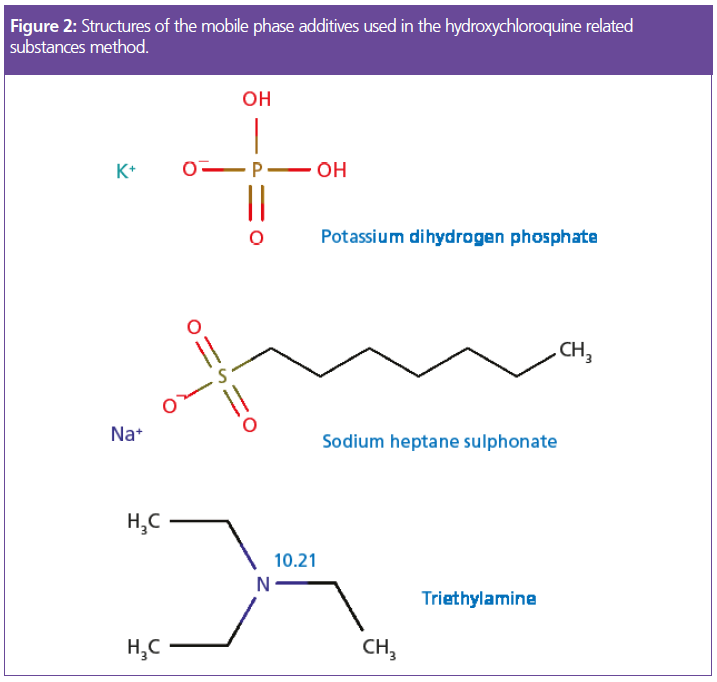
Apart from its use to obtain the correct mobile phase pH, triethylamine is used as a silanol group masking reagent. Figure 3 can give us further insight into the mechanism and requirement for this reagent.
Species 5 in Figure 3 represents an acidic silanol group which has not been effectively treated with an end capping reagent (such as that shown in species 8) which will be anionic under the mobile phase conditions of our method. If left untreated, these silanol anions will form electrostatic attractions with cationic analyte molecules and unwanted secondary interactions can lead to issues with peak broadening and tailing as well as extended column equilibration times and possible issues with peak area reproducibility. Therefore, ionogenic masking reagents such as triethylamine were traditionally added to mobile phases to effectively “end-cap” anionic surface sites, as the triethylamine, which is fully protonated below pH 7.2, is electrostatically attracted to the ionised surface silanol residues.
So, given the analytes of interest and the eluent pH, this mobile phase system would seem to be very well designed to overcome any issues with analyte reproducibility of analyte retention and peak shape. So far so good.
The Column
Let’s now look at the column being used.
The so-called bridged ethylene hydride columns were introduced by Waters in around 2004 in order to fulfil several requirements, including increased ruggedness under UHPLC conditions and increased resistance to extremes of eluent pH (especially high pH), using organic, and inorganic hybrid substrates.
However, we must go back even further to highlight some of the issues with the method under consideration. The lone (acidic) silanol groups which are masked using the triethylamine additive, were particularly prevalent when manufacturers used Type A silica as stationary phase substrate materials. This silica contained metal ions which are known to “activate” surface silanol groups towards interaction with basic analytes, and was not treated to avoid the presence of lone silanol groups. The later development of Type B silica effectively reduced silanol activity by removing target metal ions from the silica matrix and used surface treatments to reduce the number of lone silanols and promote the amount of inter-hydrated or vicinal silanol groups (species 2 in Figure 3). These developments were very effective at reducing the interactions between basic analytes and surface silanol species, effectively negating the requirement for masking reagents such as triethylamine. The bridged ethylene phases (species 1 in Figure 3) are known to further reduce the number of inherent silanol species capable of interaction with basic analytes in their ionised state.
Given the fact that, due to these new stationary phase materials, the use of silanol masking reagents have not been required for over 20 years now, one must therefore question why triethylamine is included in this method? Further, triethylamine is relatively volatile, and will be lost from the mobile phase to the atmosphere on standing, which may give us a clue as to the reason behind the drift in analyte retention over time as the inherent pH of the mobile phase will change as the triethylamine concentration lowers. In my own experience, it is also possible for the triethylamine to “settle” within the eluent, again leading to drifting or changing analyte retention. Further problems with this reagent also exist. As it inherently alters the stationary phase surface, one has to be mindful when equilibrating the HPLC column as extended flushing of the phase is often necessary to obtain reproducible retention times for the first injections of a sequence. One also must question the possible interaction between the anionic ion pairing reagent and the triethylamine cation. The possibility of this interaction, forming a neutral complex, may render the masking reagent ineffective and one needs to wonder what robustness issues this may create.
The Ion Pairing Agent
Let’s now consider the role of the ion pairing reagent in this separation. Modern stationary phases such as those used in this method, are capable of withstanding high pH mobile phase environments and as such we must also call into question the use of this type of buffer in the method. Given the pKa values of the analytes shown and their logP(D) values, it is conceivable that, by adjusting the mobile phase pH to pH 10.5 or 11, the degree of ionisation of some of the analyte basic moieties will be reduced and therefore retention may be gained, even using the more hydrophobic C18 stationary phase. Further, there are many stationary phases available which contain more polar functional chemistry or are designed to work with very little or no organic modifier within the mobile phase at the beginning of the mobile phase gradient. The use of ion paring reagents such as sodium heptane sulphonate has not been necessary for many years, since the introduction of higher quality silica, combined with more polar (so-called Polar Embedded) and more pH-resistant stationary phases can be used to gain sufficient reversed-phase retention, even for more polar analytes. Less experienced or younger chromatographers may be puzzled by the use of this reagent under these circumstances.
The Buffer Reagent
Lastly, let’s consider the use of the buffer reagent with the current column and method conditions. The column contains 1.7 μm particles which are designed for use with UHPLC systems, given the higher inherent back‑pressure created when using such small diameter particles. Intrinsically, these systems have very small internal volumes and very narrow internal diameter tubing and connectors, to reduce the effects of extra column (bed) band broadening on the separation. The use of a solid, involatile buffer with UHPLC systems is not recommended as any precipitation of the buffer through the use of highly organic eluents or via evaporation of the mobile phase on standing, will lead to significant problems with system blockages and over pressure situations. Further, the column end frits used with UHPLC columns containing 1.7 μm particles typically have a porosity of around 0.2 μm which renders them even more susceptible to blockages with solid particles. It is possible to filter the eluent solution to remove any particulate materials initially present in the eluent system, however one must again be very mindful that the triethylamine additive is relatively volatile and therefore this may be partially lost if filtration is achieved using vacuum apparatus. The target eluent pH of 7.0 is also relatively problematic, given that the pKa of at least one of the ionogenic analyte functional groups in each of the analytes is close to 7.0, we might expect a relatively large change in the ionic character of the analyte (and therefore the analyte LogD value and its polarity), unless the pH of the mobile phase is very accurately controlled each time it is made. Anyone studying robustness in undergraduate chromatography studies will be warned to stay away from the analyte pKa values to optimize the method reproducibility, due to the potential for large variations in analyte retention with relatively small changes in eluent pH. At the very least, it would surely have been better to quote a volume or weight of triethylamine required to achieve the desired pH, which could be controlled much more reproducibly.
Analysing Idiosyncracies
From this brief review it would very much appear that the current method has been “stitched together” using a very traditional mobile phase with a modern stationary phase which should not require any of the now “exotic” mobile phase additives in order to achieve a suitable separation. Without the requisite experience in critical evaluation of chromatography methods, this combination may be baffling and the resulting problems very difficult to interpret or overcome.
However, this is not the end of the story. There are other idiosyncrasies of the method which require some further thought, and which highlight some more general principles in chromatography.
On studying the solubility of hydroxychloroquine and impurities B and C, I can see no evidence of limited solubility in aqueous media, although at this point, I should declare that I’ve not undertaken any further study on the solubility of the remaining five impurities. It seems strange that the pH of the sample diluent solution is adjusted to approximately 2.8, presumably to improve the aqueous solubility by protonating the analyte molecules, but then to dissolve in 50:50 aqueous organic seems very strange. Given that the mobile phase starting composition is just 10% organic, the peak shape of the analytes will be poorer than it needs to be as the diluent composition will give a more highly eluting local environment as the sample plug enters the chromatographic bed, typically leading to peak tailing and/or splitting. There may be explainable mitigation in the solubility of the other impurities, however the sample diluent composition does seem rather counter intuitive.
The issues of equilibration have not been highlighted in the method details and, in modern times, this could be a cause of much consternation with practical application of this method. As explained above, the initial equilibration of a new column will be much longer than perhaps is usual, as there are at least two reagents which need to interact with the stationary phase to a steady state. Granted, the internal volume of the column may be relatively low (approximately 120 μL), it may take many tens if not hundreds of column volumes of mobile phase to properly equilibrate this column. The requirement for extended re-equilibration between sample injections may also be extended (see next paragraph). Further, as we are extensively modifying the stationary phase surface with the luent additives, it is good practice to dedicate the column to use only with this specific mobile phase, to avoid the requirement for extended column flushing and re‑equilibration.
It should be further noted that the detection is at a fixed wavelength of 2254 nm and this can be problematical when the ionic strength of the mobile phase changes to such a large extent during the analysis, as is the case here, where the aqueous component ranges from 90% to 15% during the course of the analysis. Without the judicious set‑up of the detector, for example by employing a reference wavelength, such changes in ionic strength might well give rise to retention time shifts, peak shape changes and unstable baselines, unless sufficient time is given to allow re-equilibration of the column between sample injections. It may be far more judicious to use a ternary system in which the ionic strength is maintained using a third eluent system which contains the buffer and additives at a higher concentration (that is, all reagents at 10× concentration and added at a constant 10% of mobile phase composition during the analysis).
I haven’t begun to address the carry‑over issues mentioned earlier, as these may well be system- rather than method-related.
In summary, this particular method appears to be sub-optimal and could be very difficult to implement under practical circumstances by less experienced users. I should also say that I’m aware that there is a new hydroxychloroquine related substances method in draft which is due for introduction in June of 2021.

Separating Impurities from Oligonucleotides Using Supercritical Fluid Chromatography
February 21st 2025Supercritical fluid chromatography (SFC) has been optimized for the analysis of 5-, 10-, 15-, and 18-mer oligonucleotides (ONs) and evaluated for its effectiveness in separating impurities from ONs.













