Developments in Field‑Flow Fractionation Coupled to Light Scattering
Field-flow fractionation (FFF) coupled to light scattering is a powerful method to separate and characterize nanoparticles, proteins, and polymers from a few nanometres to a few micrometres. The technique is one of the few that can cover the full size range of nanomaterials and provide high-resolution size distributions and additional characterization. New developments in FFF enhance performance and productivity.
rost9/stock.adobe.com
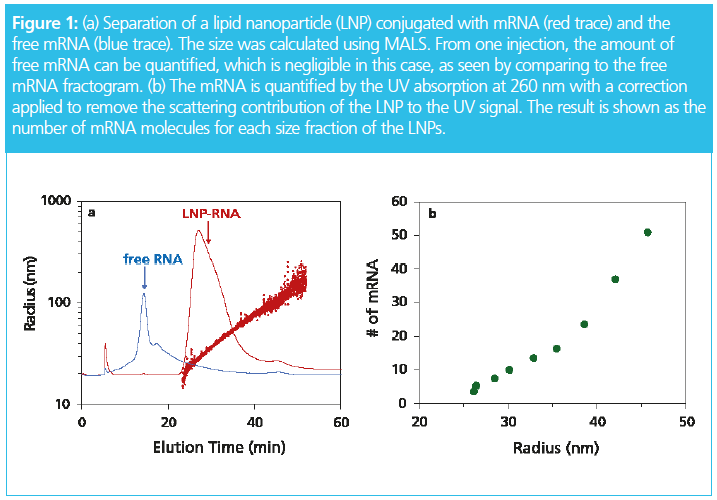
Asymmetrical flow field-flow fractionation (AF4) with multi‑angle light scattering (MALS) detection has unique benefits and has gained recognition in pharmaceutical science and technology for the characterization of vaccines, nano‑drug delivery systems, and gene vectors. In a recent publication by Jeff Clogston et al. from the US National Institutes of Health (NIH)/Nanotechnology Characterization Laboratory, the authors state that field‑flow fractionation (FFF) “is proven to be a very versatile technique and can provide a wealth of information on a material’s polydispersity and stability” (1). Figure 1 shows an example of how the ribonucleic acid (RNA) load of a lipoplex can be characterized in one injection.
Other important applications for FFF are the analysis of nanoparticles in the environment, high-molar-mass polymers used in car tyres and paints, and biopolymers, such as starch and polysaccharides, which are common food components (2,3,4).
Why has FFF not arrived in every laboratory? Certainly, every researcher in need of high‑resolution characterization of nanomaterials should be using it.
The barrier to wider acceptance is the perceived complexity of FFF: it has gained a reputation for being a complex technology that is complicated to use, with method development being seen as difficult and time consuming. Another problem is sample dilution leading to compromised detectability which hampers the application of FFF for the detection of trace components. In the case of limited sample amount, high dilution limits the utility of collected fractions for further off-line use.
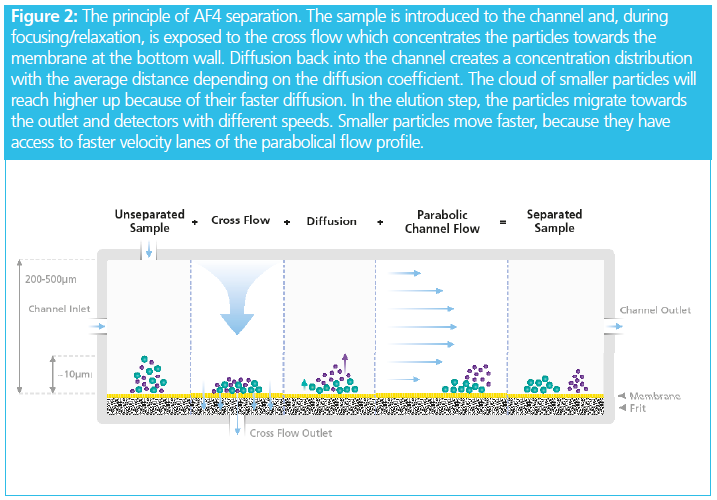
Complexity arises from the nature of FFF, which requires a set of different flow paths to be combined in a precise and controlled way. From Figure 2 it can be seen that channel flow, injection flow, cross flow, and the detector flow each play an important role in the experiment and need to be tightly regulated. Deviations will lead to lack of reproducibility in retention time or, in more extreme cases, might even cause a complete failure of the separation.
Reducing Complexity by Self‑Diagnostic Features and System Monitoring
Users of traditional FFF instrumentation must actively check flow rates and apply their expertise to detect possible deviations. In most cases, this happens only after an experiment was unsuccessful, and time and sample were lost. In terms of sample dilution there has previously been no satisfactory solution available to avoid this in routine use without the danger of sample loss and adding still more complexity to the system.
A new approach has been developed to address these bottlenecks. The novel approach is to monitor all the parameters that influence the separation in real time and implement software intelligence to perform auto diagnostic functions so that the system status can be reported. System traces are recorded as well as detector signals. These include all flow rates, the channel and trans‑membrane pressure, and the temperature of the channel and controller chassis. The software helps to check on the quality of experiments by creating an overlay view of system traces across a sample sequence. For example, a build‑up of pressure in the channel due to clogging or deviations in the injection flow rate are clearly visualized. By combining the readings of the sensors with intelligent feedback, diagnosis and troubleshooting is possible.
Experimental
The FFF–MALS system comprised an Eclipse FFF instrument equipped with DCM dilution control module, a DAWN MALS detector and an Optilab refractive index detector (all from Wyatt Technology). A 1260 Infinity II quaternary pump, vial sampler, and variable wavelength UV detector (Agilent Technologies) was used. A 240-mm-long, temperature-regulated stainless-steel FFF channel was used with a 350 µm spacer and a 10 kDa regenerated cellulose membrane (Wyatt Technology). Pierce BSA (Thermo Scientific) and a proprietary exosome sample were run in phosphate‑buffered saline, pH 7.2 with 50 mM NaCl.
Reducing Sample Dilution
In FFF, the sample is concentrated in a thin layer (typically 10 µm) at the membrane throughout the elution process. The volume above the sample layer is free from sample. At the outlet of the channel, the sample layer is mixed with the channel volume leading to a dilution which reduces detector signals.
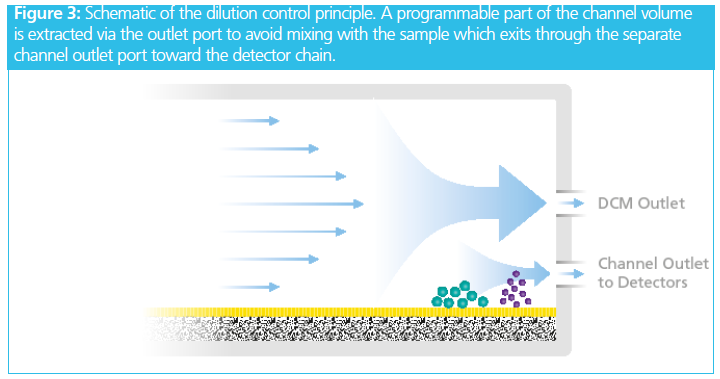
The system contains a dilution control device that extracts a programmable part of the sample‑free volume via a separate outlet port, upstream of and above the channel outlet (Figure 3). The detector flow rate is reduced in proportion to the extraction flow. If the channel flow rate is 1 mL/min and the extraction flow rate is set to 0.9 mL/min, then at the remaining detector flow rate of 0.1 mL/min, the result is a concentration enhancement of 10×. While the maximum enhancement factor is 20×, Figure 4 shows the highest ratio that maintains good analytical resolution is 5×.
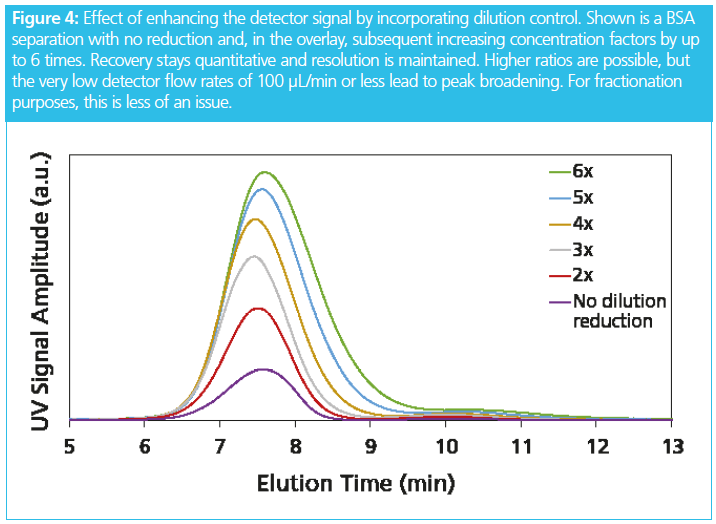
This leads to the main innovation of the implementation of dilution control. The detector flow is the residual of the main pump flow entering the channel, the cross flow, and the split flow at the extraction port. The ratio of inlet flow rate and detector flow rate can be as large as 50. The laws of error propagation dictate that the detector flow rate becomes poorly determined. Even if these flows are highly precise, even to within 0.1% the detector flow rate can deviate by 100% or more. To overcome this serious drawback, the dilution control system measures the detector flow rate and actively regulates the extracted volume to keep the detector flow rate constant. The result is a precision of better than 0.1%, leading to highly reproducible retention times as an additional benefit of the dilution control mechanism.
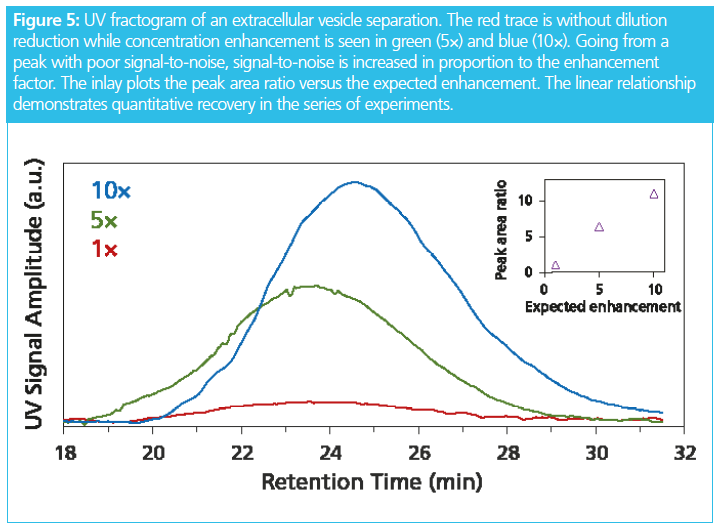
Extracellular vesicles (EVs) are typically extracted from biological samples at low concentration and sample volume may be limited as well. There is much interest among EV researchers to isolate and purify EV fractions. Although FFF is suitable for creating the size fractions, dilution and reproducibility have previously been limiting factors. As shown in Figure 5, this can now be overcome by using active dilution reduction. A 10 times increase in concentration can be achieved at quantitative recovery. In conjunction with a semi‑preparative FFF channel, milligram quantities of EV fractions can be produced.
Summary
A new type of dilution control mechanism has been developed to increase the sample concentration at the detector up to a factor of 10. This helps to quantify trace amounts of sample components, analyze precious samples that cannot be obtained in larger quantities, and makes fraction collection more effective.
Instrument and software developments in FFF, specifically for AF4, have also been developed that decrease the complexity of operating the FFF system. This is achieved by adding multiple sensors to the hardware and adding software that digests the data to perform self‑diagnostic functions and actively support the user to troubleshoot problems and maintain the system.
Reference
- Y. Hu, R.M. Crist, and J.D. Clogston, Anal. Bioanal. Chem. 412, 425–438 (2020).
- S. Podzimek, J. Machotova, H. Zgoni, P. Bohacik, and J. Snuparek, Polym. Plast. Technol.Eng. 55(13), 1365–1372 (2016).
- Q. Chevalier, H. El Hadri, P. Petitjean, M. Bouhnik‑Le-Coz, S. Reynaud, B. Grassl, and J. Gigault, Chemosphere 194, 125–130 (2018).
- A. Castro, F. Vilaplana, and L. Nilsson, Food Chem. 223, 76–81 (2017).
Christoph Johann earned his PhD in Physical Chemistry at the University of Mainz, Germany, in 1985. He has been active in polymer and biopolymer analysis for over 30 years and has several peer-reviewed publications in the field of macromolecular characterization. In 1991 he introduced commercial field-flow fractionation systems in Europe. In 1993 he founded Wyatt Technology’s main European subsidiary. He has been global product manager for Wyatt since 2018.

Retaining Talent in Field-Flow Fractionation: An Initiative
The authors present their motivation for establishing the Young Scientists of FFF (YSFFF) initiative within the FFF community.
Field-Flow Fractionation: Virtual Optimization for Versatile Separation Methods
August 8th 2017Flow-field flow fractionation (flow-FFF) offers highly versatile separations for the analysis of complex fluids, covering a size range of macromolecules and particles from 1 nm to 10,000 nm. However, flow-FFF is often perceived as a difficult technique to learn because of the multiple parameters available for adjustment. Recent advances in software for simulating flow-FFF overcome this obstacle, enabling the virtual optimization of flow-FFF methods and opening up the power of flow-FFF separations to non-experts. An added benefit is the ability to easily analyze particle size distributions by elution time from first principles.









