HPLC: Continuing to Answer Challenges to Food and Drug Safety
LCGC North America
This month's installment of "Innovations in HPLC" discusses the use of HPLC for these recent threats to health and safety.
Bisphenol A (2,2'-bis[4-hydroxyphenyl]propane), or BPA, is a key industrial chemical used to make polycarbonate plastic and epoxy resins and is used to prevent food contamination in canned goods and other food packaging. BPA can be released from these materials and can induce adverse effects in humans including hormonal responses, because it mimics estrogen. BPA is consistently in the news as mothers worry about it leaching from their baby bottles and hikers from their water bottles. Regulatory bodies like the FDA and county, state, and national legislatures are grappling with outright bans, or the existence of true causal relationships and effects and the BPA level at which the effects gain significance.

Michael Swartz
In early 2008, unusual reactions to heparin, a highly sulfated glycosaminoglycan widely used as an injectable anticoagulant, prompted a recall of the drug product later found to be adulterated. In another case of adulteration dating back to 2007, melamine added to food products to increase the apparent protein content was found to cause severe health issues.
In two of these three cases, high performance liquid chromatography (HPLC) played a significant role in discovery, and in all three cases, HPLC continues to play a role in subsequent monitoring and analysis. In this month's column, I'll discuss these three unique applications of HPLC, and how HPLC technology has helped to answer the challenges presented.
BPA: From Baby Bottles to Minestrone
BPA (Figure 1a) was first synthesized over 100 years ago and has been in commerce for more than 50 years (1). It is used in the synthesis of polyesters, polysulfones, and polyether ketones as an antioxidant in some plasticizers, and as a polymerization inhibitor in PVC. It is a key monomer in production of polycarbonate plastic and epoxy resins, and it is used in consumer products as diverse as baby and sport water bottles, sports equipment, medical and dental devices, bicycle helmets, lenses, household electronics, and CDs and DVDs. It is also used as a fungicide, in beverage and food can coatings, and is a precursor to the flame retardant tetra-bromo-bisphenol A. Plastic products and epoxy resins made from BPA monomers are classified as Type 7 (sometimes referred to as the catch-all "other" class) plastics, and BPA was approved by the FDA for use in food contact materials in the 1960s.
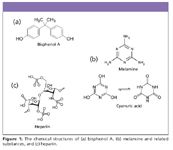
Figure 1
All good, right? Not so fast. In recent years, new concerns have been raised about BPAs safety. As long ago as 1938, BPA was discovered to be an artificial estrogen, and it has long been considered as an endocrine disruptor (2–4). In September 2008, the National Toxicology Program of NIH determined that BPA might pose risks to human development, raising concerns for early puberty, prostate effects, breast cancer, and behavioral impacts from early-life exposures (5). In the interim, conservative estimates of upwards of 100 peer-reviewed papers have been published studying the affects of BPA from a variety of sources, including both food-related and environmental (1,6–9). As a result, the EPA (10) and the FDA (11) have each weighed in on the topic, and the FDA, as a result of a recent assessment, released a draft report finding that BPA remains safe in food contact materials. This position is being re-assessed, however, as a subcommittee of FDA's science board recently raised questions about whether FDA's review had adequately considered the most recent scientific information available (12). That report is due out by the end of 2009, so by the time you read this, additional guidance on BPA exposure might be available from the FDA. Additional information about BPA is also available from the FDA website (13).
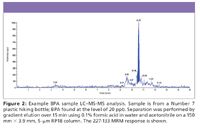
Figure 2
Both HPLC and gas chromatography (GC) have been used for the analysis of BPA, with a variety of detectors including ultraviolet (UV), fluorescence, and mass spectrometry (MS). HPLC generally is preferred over GC for better accuracy and precision and faster run times (14). Reversed-phase HPLC using C18 columns and buffered (for example, 0.1% formic acid) or unbuffered aqueous–organic mobile phases containing either methanol or acetonitrile have been used. The choice of detector depends upon the desired level of detection (often into the low parts-per-billion [ppb] range), accuracy and precision, and the specificity needed given the sample matrix. For many analyses, UV or fluorescence detectors might suffice, particularly because sample preparation as outlined in Table I often results in a 1–2 orders of magnitude preconcentration (14,15). Assays with UV and fluorescence detectors typically provide the best accuracy and precision, however, MS or MS-MS detection using electrospray ionization (ESI) in negative ion mode provides the best specificity and sensitivity, albeit at a cost (16,17). An example MS-MS analysis of BPA from a commercially available water bottle using the sample preparation method outlined in Table I is illustrated in Figure 2. Recoveries are routinely above 90%, and repeatability is < 1% to a high of 7% for the method, depending upon the level.

Table I: BPA sample preparation
Melamine in Pet Food and Infant Formulas
Melamine is a nitrogen-based compound that is characterized by its high nitrogen content. It is also known by other chemical names, such as triaminotriazine, cyanuramide, and cyanuric acid amide. Melamine is an industrial chemical used in the manufacturing of resins for surface laminates and adhesives in the production of wood-based panels. Melamine and its resins also are used in making melamine dinnerware, additives for textiles, and as flame-retardant additives for foam mattresses. Cyanuric acid is structurally related to melamine and is sometimes found as a melamine impurity. The structures of melamine and cyanuric acid are shown in Figure 1b.
In 2007, the FDA learned that some pet foods were responsible for the sickness and deaths of cats and dogs. It was found that vegetable proteins imported into the U.S. from China and used as ingredients in pet and livestock food products were contaminated with melamine and cyanuric acid. Either of these two compounds possess limited toxicity individually; however, when they were ingested together, a crystallized precipitate was formed in the animals' kidneys, causing renal failure. Soon after, in September of 2008, the FDA received reports of melamine-contaminated infant formula in China. Melamine apparently was added to increase the apparent protein content (and consequently the market value) in foods or in ingredients used in processed food products intended to contain protein.
Food protein typically is measured by a method developed by Danish brewer Johann Kjeldahl in the late 1800s. Kjeldahl analyses use a strong acid to digest samples to release nitrogen, which is then converted to ammonia. The amount of ammonia indicates how much nitrogen was in the original sample, corresponding to the amount of protein. Another, similar nitrogen-based technique, called the Dumas test, is also popular with industry. It relies on burning the sample to release nitrogen. Because both the Kjeldahl and Dumas tests estimate protein levels by measuring the nitrogen content, artificially high results can be obtained by adulteration of food products with nitrogen-rich compounds such as melamine.
When the pet-food scandal broke, an established analytical technique for detecting both melamine and cyanuric acid in consumer products down to the required 1-ppm level did not exist and chemists had to respond quickly to devise new analytical techniques to address melamine adulteration. The FDA consequently developed three analytical methods, one that is based upon GC–MS, and two that are based upon LC–MS-MS.
The GC–MS method is described in the FDA Laboratory Information Bulletin No 4423 and can be used to screen for the presence of melamine, ammelide, ammeline, cyanuric acid, and 2,6-diamino-4-chloropyrimidine (internal standard) in a variety of matrices at a minimum reporting level (MRL) of 10 µg/g (ppm) and above (18). Samples are extracted using a mixture of acetonitrile–water–diethylamine and the analytes are subsequently converted to trimethylsilyl derivatives for analysis.
The LC–MS-MS methods are described in FDA Laboratory Information Bulletins No 4421 and 4422 (19,20). Developed for the analysis of residues of cyanuric acid and melamine in infant formula, Method 4421 uses an initial extraction with 2.5% aqueous formic acid, followed by a series of filtration, centrifugation, and dilution steps. Both Method 4421 and Method 4422 use a common zwitterionic hydrophilic interaction chromatography (HILIC) column for the analysis of both compounds. ESI is used in both the negative ion (cyanuric acid) and positive ion (melamine) modes, and two selected reaction monitoring (SRM) transitions are monitored for each compound. Method 4421 is capable of a limit of quantitation (LOQ) down to 0.25 µg/g (250 ppb) for both analytes. Example data from Method 4421 is shown in Figure 3.

Figure 3
With Method 4422, both cyanuric acid and melamine can be extracted from tissue and infant formula with a 50:50 acetonitrile–water extraction solution, followed by centrifugation. The cleanup procedure for melamine involves a mixed-mode cation exchange solid-phase extraction (SPE), and for cyanuric acid, a mixed-mode anion exchange SPE. Aliquots of the same extract are processed individually, and the final extracts for both melamine and cyanuric acid are in acetonitrile, making the procedure amenable to evaporating the excess solvent for preconcentration or solvent exchange if needed. As with Method 4421, Method 4422 uses a common zwitterionic HILIC column and LC method for the analysis of both compounds. The MS methods are nearly identical for both 4421 and 4422, however, the latter uses a commercially available, isotopically labeled internal standard for each compound to correct for any matrix effects, responsible in part for lowering the LOQ to 10 ppb.
The FDA methods for melamine have been adapted for use on several different vendors' instrumentation, including ultrahigh-pressure liquid chromatography (UHPLC) (21,22). HPLC–UV methods that avoid the use of derivatization and MS detection also have been reported (23). The HPLC–UV method uses a mixed-mode hydrophobic–weak cation exchange column for the analysis of melamine and reported a limit of detection in the sub-part-per-million range.
In addition to food safety considerations, the FDA also has issued a guidance regarding pharmaceutical components at risk for melamine contamination (24). The guidance is intended to alert pharmaceutical manufacturers of finished products, pharmacy compounders, repackers, and suppliers to the potential risk of melamine contamination in pharmaceutical components. The components considered to be at-risk for melamine contamination are listed in Table II. These components were identified by the FDA from a search of USP monographs and the FDA Inactive Ingredient Database. For each compendia component listed, a test for total nitrogen content is specified in the USP monograph. The USP method for total nitrogen content is based upon a titration method (USP Chapter <461>). However, alternative methods also can be used in screening components for the presence of melamine, as long as the method is capable of detecting melamine contamination down to at least 2.5 ppm. The earlier incidents with pet food and infant formula illustrate the potential for drug components to be contaminated with melamine. Therefore, it is important for drug manufacturers to be diligent in ensuring that no component used in the manufacture of any drug is contaminated with melamine.
Heparin Adulteration
Heparin is a highly sulfated glycosaminoglycan widely used as an injectable anticoagulant (blood-thinning) drug. Its structure is shown in Figure 1c. In early 2008, adverse reactions, serious injuries, and deaths were associated with the use of heparin, traced to lots that contained active pharmaceutical ingredient (API) from China.
After launching a far-ranging investigation, FDA scientists established that the heparin API had been adulterated with a highly sulfated version of chondroitin sulfate (a galactosamine glycosaminoglycan), now referred to as oversulfated chondroitin sulfate (OSCS). OSCS is a less costly substance that can mimic the blood-thinning properties of heparin (25,26).
At the time, the assay for heparin sodium in the USP monograph measured heparin anticlotting activity but could not detect the synthetic OSCS (20). As a result, the USP revised the monograph in two stages starting in June of 2008. In the Stage 1 revision, USP 32, the immediate health issue was addressed by identifying OSCS in heparin by capillary electrophoresis (CE) (28). Based upon the public comments received from the Stage 1 revision, the USP replaced the CE method with an anion-exchange chromatography identification method in the Stage 2 revision. The chromatography method improves the resolution of heparin from impurities, such as OSCS and dermatan sulfate (29). In addition, the USP added a method using high-performance anion-exchange with pulsed amperometric (electrochemical) detection (HPAE–PAD) to identify and quantitate the level of organic impurities. The Stage 2 monograph was implemented in October 2009 and the standards are now enforceable in the U.S. by FDA.

Figure 4
The heparin chromatographic identity section of the new USP monograph, used to determine OSCS and dermatan sulfate in heparin, uses an anion-exchange column (USP L61-type) and a sodium phosphate–sodium perchlorate gradient separation. An example separation is illustrated in Figure 4. The broad heparin peak can be attributed to the distribution of heparin chain lengths and the variation in the number of sulfate groups per molecule. This method takes advantage of the hydrophilic character and low capacity of the ion-exchange stationary phase to separate 1% (v/v) concentrations of dermatan sulfate and OSCS from the broad single heparin peak (30).
The organic impurities section of the new heparin USP monograph relies upon hydrolyzing the polysaccharide and determining the relative amounts of galactosamine and glucosamine in the sample digests by analysis on an anion exchange column with electrochemical detection. Heparin is composed of glucosamine and uronic acid and acid hydrolysis of heparin samples releases the glucosamine, which is determined readily with high sensitivity by pulsed amperometric electrochemical detection (PAD). In comparison, OSCS is composed of galactosamine and uronic acid. In these compounds, acid hydrolysis releases galactosamine, also determined easily by electrochemical detection. The USP method measures the ratio of galactosamine/glucosamine as an indication of the heparin purity and to identify heparin samples that might be contaminated or adulterated with chondroitin sulfate compounds. An example of the separation is shown in Figure 5. The combination of the anion exchange separation and the sensitivity of PAD allows for the detection of galactosamine at levels as low as 0.4% (31).

Figure 5
Conclusion
HPLC continues to rise to the occasion time after time to solve some of the most challenging issues facing chemists in the laboratory today. In the case of melamine and heparin, HPLC has been used to solve important health problems quickly, and prevent further injury and even deaths from occurring from the adulteration of our food and drug supplies. For BPA analysis, HPLC will continue to play a role in the years to come as toxicologists, environmentalists, legislators, and concerned parents continue to study the affects of BPA on human health and the environment.
Acknowledgments
The author would like to acknowledge the assistance of Adam Grenier of Synomics Pharma for his contribution to the BPA work cited in this article.
Michael Swartz "Innovations in HPLC" Editor Michael E. Swartz is Research Director at Synomics Pharmaceutical Services, Wareham, Massachusetts, and a member of LCGC's editorial advisory board. Direct correspondence about this column to "Innovations in HPLC," at lcgcedit@lcgcmag.com
References
(1) See: http://www.ewg.org/reports/bpatimeline
(2) E.C. Dodds and W. Lawson, Proc. Royal Soc. London B 125, 222–232 (1938).
(3) I. Rykowska, and W. Wasiak, Acta Chromatographica 16, 7–27 (2006).
(4) H.H. Le, E.M. Carlson, J.P. Chua, and S.M. Belcher, Toxicol. Lett. 176, 149–156 (2008).
(5) See: http://cerhr.niehs.nih.gov/chemicals/bisphenol/BPADraftBriefVF_04_14_08.pdf, or NIH publication No. 08-5994.
(6) R.A. Trenholm, B.J. Vanderford, J.E. Drewes, and S.A. Snyder, J. Chromatogr., A 1190, 253–262 (2008).
(7) I. Brossa, Pocurull, F. Borrull, and R.M. Marce, Chromatographia 56, 573–576 (2002).
(8) A. Latorre, S. Lacorte, and D. Baarcelo, Chromatographia 57, 111–116 (2003).
(9) J-P. Baugros, B. Giroud, G. Dessalces, M-B. ier-Loustalot, and C. Cren-Olive, Anal. Chim. Acta 607, 191–203 (2008).
(10) See: http://cfpub.epa.gov/ncea/iris/index.cfm?fuseaction=iris.showQuickView&substance_nmbr=0356
(11) See: http://www.fda.gov/ohrms/dockets/AC/08/briefing/2008-0038b1_01_02_FDA%20BPA%20Draft%20Assessment.pdf
(12) See: http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4386b1-05.pdf
(13) See: http://www.fda.gov/Food/FoodIngredientsPackaging/ucm166145.htm
(14) M. Swartz and A. Grenier, "Sample Preparation and Chromatographic Analysis of Bisphenol A", presentation at Eastern Analytical Symposium (EAS), Somerset, New Jersey, November 2009.
(15) C. Nerin, C. Fernandez, C. Domeno, and J. Salafranca, J. Agr. Food Chem. 51, 5647–5653 (2003).
(16) M.S Diaz-Cruz, M.J Lopezde Alda, R. Lopez, and D. Barcelo, J. Mass. Spectrom. 38, 917 (2003).
(17) A. Lagan A. Bacaloni, I. De Leva, A. Faberi G. Fago, and A. Marino, Anal. Chim. Acta 50, 179 (2004).
(21) Waters Application Note 720002889en, January 2009. Waters Corporation, Milford, MA or www.waters.com
(22) Waters Application Note 720002865en, December 2008. Waters Corporation, Milford, MA or www.waters.com
(23)Dionex Corporation, Application Note 221, LPN 2181. Dionex Corporation, Sunnyvale CA, 2009.
(24) See: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM175984.pdf
(25) FDA Press Release, "Baxter's Multiple-Dose Vial Heparin Linked to Severe Allergic Reactions. Federal Drug Administration," February 11, 2008.
(26) M. Guerrini, D. Beccati, Z. Shriver, A. Naggi, K. Viswanathan, A. Bisio, I. Capila, J.C. Lansing, S. Guglieri, B. Fraser, A. Al-Hakim, N.S. Gunay, Z. Zhang, L. Robinson, L. Buhse, M. Nasr, J. Woodcock, R. Langer, G. Venkataraman, R.J. Linhardt, B. Casu, G. Torri, R. Sasisekharan, Nat. Biotechnol. 26, 669–675 (2008).
(27) Heparin Sodium monograph in The United States Pharmacopeia 31, Supplement 2, NF 26; American Pharmaceutical Association, Washington, DC, 2008.
(28) Heparin Sodium monograph in The United States Pharmacopeia 32, Supplement 2, NF 25; American Pharmaceutical Association, Washington, DC, 2009.
(29) Heparin Sodium, Pharmacopeial Forum 35(2), 1–10 (2009).
(30) Dionex Corporation, "Determination of Oversulfated Chondroitin Sulfate, Dermatan Sulfate, and Heparin Sodium Using Anion-Exchange Chromatography with UV Detection (IC-UV)," Application Note 235, LPN 2306. Sunnyvale, California, 2009.
(31) Dionex Corporation, "Determination of Galactosamine Containing Organic Impurities in Heparin by HPAE-PAD Using the CarboPac PA20 Column," Application Note 233, LPN 2286, Sunnyvale, California, 2009.

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.
Characterizing Polyamides Using Reversed-Phase Liquid Chromatography
May 5th 2025Polyamides can be difficult to characterize, despite their use in various aspects of everyday life. Vrije Universiteit Amsterdam researchers hoped to address this using a reversed-phase liquid chromatography (RPLC)-based approach.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












