HPLC Column Standardization in Pharmaceutical Development: A Case Study
LCGC Asia Pacific
Significant benefits can be obtained by standardizing high performance liquid chromatography (HPLC) columns in a pharmaceutical development laboratory. Here is a story of how one organization attempted to encourage its staff to develop HPLC methods using fewer column brands and dimensions to reduce waste and efforts in method transfers downstream.
Michael W. Dong, Perspectives in Modern HPLC Editor
Significant benefits can be obtained by standardizing high performance liquid chromatography (HPLC) columns in a pharmaceutical development laboratory. Here is a story of how one organization attempted to encourage its staff to develop HPLC methods using fewer column brands and dimensions to reduce waste and efforts in method transfers downstream.
A typical pharmaceutical company may use hundreds or even thousands of high performance liquid chromatography (HPLC) columns each year. This diversity in column dimensions, manufacturers, particle sizes, and bonded phases reflects the preferences of method developers in the organization. In turn, these preferences continue their downstream propagation as methods are transferred to quality control and global manufacturing laboratories (1,2). This freedom of choice may not be the best thing for a global organization because this proliferation of brands, phases, and column geometries adds a considerable amount of cost and wasted efforts for the organization.
With advances in new HPLC and ultrahigh-pressure liquid chromatography (UHPLC) column technologies such as high-purity silica, silica-hybrid particles, smaller and monodispersed particles, superficially porous particles (SPPs), and novel bonded-phase chemistries, there are numerous column choices for method developers (3–6). Nonetheless, selection of the best or most appropriate columns for an intended use does not always happen, because each method developer has his or her unique experience and preferences. Many researchers have their own preferred vendors and column phases from their previous experience. In addition, the column selection process for a method development project is often hurried, allowing no more than a few weeks for an analytical chemist to develop a reasonable stabilityâindicating method for a drug candidate, conduct forced degradation studies, and begin initial stability studies for that new candidate (1,2). Moreover, after the method has been validated and then submitted in a regulatory filing, the chosen column is subsequently used for product release testing and stability studies, and the corresponding method is eventually transferred to other facilities such as company manufacturing plants or to contract manufacturing organizations (CMOs).
A similar sequence of method development, validation, and transfer processes occurs for analytical methods applied for the quality assessments of starting materials and critical raw materials used in the synthetic process for the drug candidate, although generic broadâgradient purity methods are often sufficient for purity evaluation of these precursor materials (7,8). For these less-demanding assessment methods, there may not be a need for a specific column from a manufacturer, which can allow an organization to achieve a tremendous cost saving by using the same common generic method or set of methods between all projects within the organization (1).
When these scenarios are multiplied by the number of drug development projects, a pharmaceutical laboratory can easily have several hundred LC columns of different brands, bonded phases, particle sizes (dp), lengths (L), inner diameters (dc), and usage histories. This “column proliferation” often causes extra technical issues in method validation or transfer and results in an inventory of hundreds of “unique” or “orphan” columns in the facility. The following is a story of how one organization attempted to ameliorate column proliferation by advocating the use of fewer “standardized columns” in the department.
A Column Standardization Technical Focus Group
The organization in this case study was an analytical chemistry and quality control department of a medium-toâlarge pharmaceutical company that supported chemistry, manufacturing, and control (CMC) in its small-molecule drug discovery programme. It had a staff of more than 50 people, including ~30 laboratory personnel consisting of scientists and research associates working in small teams to support multiple early-phase projects. All laboratory personnel were required to work in the laboratory to develop new methods for raw materials, starting materials, intermediates, drug substances, and drug products to assess purity (both chemical and chiral), stability, and other critical quality attributes.
HPLC was the primary analytical technique, and the cost of HPLC columns was a major fraction of the consumables budget. The laboratory did not use a centralized HPLC column stocking programme, and each individual scientist procured his or her own columns.
Eventually, these columns (used and unused) would wind up in laboratory bench drawers, cabinets, or individuals’ offices (see examples in Figure 1). While each individual had a secret stash of his favourite columns for active projects, hundreds of columns from completed and obsolete projects were scattered throughout the laboratory. Nevertheless, when a new project was started, one never seemed to be able to find the “right” column amidst the myriad columns available. Such a scenario would often result in last-minute group emails to the entire department asking for a specific column from a particular manufacturer. This situation reminds me of the famous quote, “Water, water, every where, nor any drop to drink” (9).
Figure 1: Pictures showing column proliferation in an overstocked cabinet, a repository for columns used in automated screening systems, a laboratory bench drawer full of used columns, and a stash of brand new columns allocated to a specific project in a personal office.

This diversity of column usage, reflecting personal preferences within the group, was not necessarily a bad thing. However, it did get worse when these methods were validated and eventually transferred to CMOs to support production of the clinical trial materials. Since column availability could be a problem for foreign CMOs, it was often necessary to stockpile more columns for those CMOs. Column proliferation probably occurs in most pharmaceutical companies, except in this case a few individuals decided to form a technical focus group to evaluate the issue and propose changes.
Scope and Goals of the Column Standardization Technical Focus Group
The first thing our technical focus group did was to discuss the scope and goals. A consensus was reached to identify a set of primary columns for use in the development of stabilityâindicating methods and another set of secondary, shorter columns for potency determination, in-process control (IPC), and other screening methods. Initially, our focus for both column sets was on achiral reversed-phase HPLC columns. Columns with limited applications using other chromatographic modes such as supercritical fluid, normal phase, ion-exchange, hydrophilic interaction, and mixed-mode would not be in the standardization programme. Chiral columns, which did constitute a major fraction of the consumables column budget, would be considered later.
The group agreed that the main objective was not to stifle creativity, but rather to find sets of “best” columns for stability-indicating methods and generic IPC–potency methods via a consensus-building process. The recommendations would include column dimensions, particle size, support type (SPPs, totally porous particles [TPPs], hybrids), as well as specific manufacturers or brands. The benefits were expected to be increased productivity (less time and effort in method development and transfer) and cost savings by purchasing columns that could be used across different projects. Another benefit was increased awareness by those in our department of the latest trends and best practices in HPLC column technology.
We began by conducting a poll of current column preferences, to be followed by a discussion on optimum column dimensions, particle diameter, bonded phase, and brands. Our first deliverables were compiled lists of recommended columns for stabilityâindicating assays and for potency–IPC assays.
The poll results came back within a few weeks with contributions from at least 20 staff. Trends and observations were tallied. From the poll, our department appeared to have preferences for columns from four manufacturers:
- The first one for its strength in hybrid particles, particularly those with a positively charged surface and its new line of columns packed with sub-3-µm SPPs.
- The second one for its C18 columns known for excellent peak shape for basic pharmaceutical compounds and batch-to-batch reproducibility for quality control applications. This manufacturer also introduced a new set of bonded phases with unique selectivity.
- The third one for its secondâgeneration hybrid and for its substantial product offerings in SPP with particle diameters ranging from sub-2 µm to 5 µm.
- The fourth one for its C18 columns with a long history of robustness and a line of innovative SPPs, including one compatible with high-pH mobile phases.
Not surprisingly, additional details from our poll indicated that 3-µm materials remained the top particle size choice, as well as an increasing preference for columns packed with sub-3-µm SPPs. The most popular inner diameter was the standard 4.6 mm, although many were shifting their preferences towards smaller 3.0-mm i.d. columns.
Recommendations by the Group
After several meetings and considerable discussions, the technical focus group reached agreement on a list of technical recommendations and two column sets (shown in Table 1). These
were presented at a departmental meeting to illicit more open feedback and discussion. Rationales for these recommendations are described in the next section.
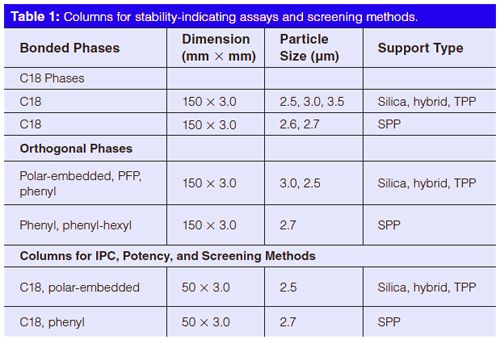
Recommended Geometries:
- 150 mm × 3.0 mm columns packed with either 3- or sub-3-µm particles for stability-indicating methods.
- 50 mm × 3.0 mm columns packed with either 3- or sub-3-µm particles for potency, IPC, cleaning verification, and other screening methods.
Recommended Phases:
- A set of C18 and “orthogonal” bonded phases (phenyl, polarâembedded, pentafluorophenyl [PFP], and cyano [CN] phases) (6) for stabilityâindicating methods. This set would be used with the automated column–mobile phase screening system in the department. A similar set for potency, IPC, and screening methods consisting of mostly 50 mm × 3.0 mm columns packed with C18, phenyl, and polaâembedded phases.
Rationales for the Recommendations Column Inner Diameter:
The 3.0âmm i.d. column was recommended over the standard 4.6âmm i.d. column for several reasons (10):
- The laboratory was equipped with both HPLC and UHPLC equipment (about 50:50) that worked well with 3.0-mm i.d. columns.
- The optimum flow rate for 3.0-mm i.d. columns packed with 3-µm materials is around 1 mL/min; in contrast, the optimum flow rate for a similar 4.6-mm i.d. column is around 2.0 mL/min with twice the solvent usage (10).
- Although 2.1-mm i.d. columns are commonly used with UHPLC systems, they are much less compatible with conventional HPLC systems from the standpoints of system dispersion and precision for small-volume injections (4).
Particle Size: The 3-µm and subâ3âµm particles were selected because they offered a good balance of efficiency and pressure requirements that were compatible with both HPLC and UHPLC equipment. Sub-2-µm particles would have significant advantages in efficiency and speed, but are less compatible with conventional HPLC equipment, especially for column lengths greater than 50 mm.
Column Length: Our group selected a standard column length of 150 mm for stability-indicating methods. A 150 mm × 3.0 mm column packed with 3- or sub-3-µm materials have column efficiencies of 20,000 to 30,000 plates (or gradient peak capacities of 200–400 within ~30 min) (11), which we considered a good match for ICHâcompliant stability-indicating methods. Similarly, 50 mm × 3.0 mm columns would offer a good balance of speed and resolution for less-demanding generic screening methods (1). A 100 mm × 3.0 mm column would also be a viable choice with intermediate speed and efficiency, although the group decided to standardize on the longer 150-mm column instead.
Superficially Porous Particles: The rationales for selecting SPPs over TPPs have been well documented in the literature, because of their superior efficiency performance versus TPPs of the same particle diameter (with reduction of reduced plate heights by ~20–40%) (12,13). With more than 20 manufacturers offering columns packed with SPPs, these materials are quickly becoming the preferred HPLC–UHPLC support for pharmaceutical analysis.
Bonded Phases: Not surprisingly, C18 remained the dominant bonded phase of choice by most of the staff because of its high hydrophobicity, retention, and batchâto-batch reproducibility (6). There was a strong preference in the department for a C18 bonded phase with a slight positively charged surface and another C18 phase on an SPP designed for high-pH mobile phases. Both bonded phases yielded excellent peak shape for highly basic analytes when used with lowâionicâstrength mobile phases (for example, 0.05% formic acid) (1,14,15).
Several “orthogonal” bonded phases with different selectivity than C18 were also selected, notably those with polar-embedded phases (amide or carbamate polar groups) and phenyl phases for their enhanced selectivity for aromatic compounds with π-π interactions (6).
Implementation
It was easy to make recommendations, but it was significantly more challenging to implement these changes to realize any real impact in the department. Since the group had no authority to mandate these proposed changes, we instituted a friendly persuasion approach by emulating casinos that entice customers with free meals and lodging. We encouraged usage of these “preferred” columns by stocking them in the laboratory. Bulk orders were negotiated with manufacturers at deep discounts, and columns were stored in a controlled location so inventory could be maintained and made immediately available when needed.
Summary and Conclusion
Although it would be difficult to quantify the impact of the column standardization programme, our rational column selection strategy had the desired effect of reducing the numerous types of columns used across the projects in the department. The use of polling, open discussions, and consensus building allowed us to compile a list of “best” columns for pharmaceutical analysis in our laboratories. We hope that our story might encourage other laboratories to consider similar programmes to reduce column spending, minimize waste, and improve laboratory productivity.
Acknowledgements
The author would like to thank members of the technical focus group on column standardization for their efforts in developing recommendations based on departmental preferences, and in the implementation of the column stocking programme. Members were Charlotte Tsang, Dawen Kou, Bob Garcia, and Tania Ng of Genentech. The author would like to thank Tom Waeghe of Mac-Mod Analytical, Xiaoli Wang of Agilent, David Van Meter of Rottendorf Pharma, and Davy Guillarme and Szabolcs Fekete of the University of Geneva, for their editorial input and suggestions.
References
- M.W. Dong, LCGC Europe29(6), 328–337 (2016).
- HPLC for Pharmaceutical Scientists, Y.V. Kazakevich and R. LoBrutto, Eds, (Wiley, Hoboken, New Jersey, USA, 2007).
- R. Majors, LCGC Europe28(12), 658–665 (2015).
- D. Guillarme and M.W. Dong, Amer. Pharm. Rev.16(4), 36–43 (2013).
- U.D. Neue, HPLC Columns: Theory, Technology, and Practice (Wiley-VCH, New York, USA, 1997).
- M.W. Dong, Modern HPLC for Practicing Scientists (Wiley, Hoboken, New Jersey, USA, 2006), Chapters 2, 3, 7.
- M.W. Dong, LCGC North Am.34(6), 408–419 (2016).
- M.W. Dong, LCGC Europe26(8), 455–461 (2013).
- S.T. Coleridge, The Rime of the Ancient Mariner, in Lyrical Ballads (1817).
- M.W. Dong, LCGC North Am.32(8), 552–557 (2014).
- D. Guillarme, E. Grata, G. Glauser, J.L. Wolfender, J.L. Veuthey, and S. Rudaz, J. Chromatogr. A 1216, 3232–3243 (2009).
- S. Fekete, D. Guillarme, and M.W. Dong, LCGC North Am.32(6), 420–433 (2014).
- S. Fekete, E. Oláh, and J. Fekete, J. Chromatogr. A 1228, 57–71 (2012).
- K.J. Fountain, H.B. Hewitson, P.C. Iraneta, and D. Morrison, “Practical Applications of CSH Technology,” Waters Corporation, Milford, Massachusetts, USA, 720003720EN, Sept. 2010.
- W.J. Long, A.E. Mack, X. Wang, and W.E. Barber, Recent Developments in HPLC and UHPLC supplement to LCGC Europe28(s6), 31–38 (2015).
Michael W. Dong is a principal of MWD Consulting, which provides training and consulting services in HPLC and UHPLC, pharmaceutical analysis, and drug quality. He was formerly a Senior Scientist at Genentech, Research Fellow at Purdue Pharma, and Senior Staff Scientist at Applied Biosystems/Perkin-Elmer. He holds a PhD in Analytical Chemistry from City University of New York. He has more than 100 publications and a best-selling book in chromatography. He is an editorial advisory board member of LCGC North America. Direct correspondence to: LCGCedit@ubm.com

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.









