UHPLC Instrument Variations and Approaches to Ease the Method Transfer Process
LCGC Asia Pacific
Ultrahigh-pressure liquid chromatography (UHPLC) instruments from different manufacturers and instruments with different configurations can produce significant variations in chromatographic separation. The variety in instrument configuration increases the complexity of the method development process, which now requires a more thorough evaluation of the effect of instrument variations on the method. The studies presented here determined the typical interinstrument variations in dwell volume, extracolumn dispersion, and mixing efficiency as measured by mobile-phase compositional accuracy. Additionally, the dwell volume and extracolumn dispersion were independently and systematically varied to evaluate the resulting impact on resolution for a small-molecule test mixture during gradient elution. To account for these interinstrument variations, dwell volume and wash-out volume method translation and adjustment techniques were evaluated.
Laura E. Blue, Tawnya Flick, and David Semin, Amgen Inc., Thousand Oaks, California, USA.
Ultrahigh-pressure liquid chromatography (UHPLC) instruments from different manufacturers and instruments with different configurations can produce significant variations in chromatographic separation. The variety in instrument configuration increases the complexity of the method development process, which now requires a more thorough evaluation of the effect of instrument variations on the method. The studies presented here determined the typical interinstrument variations in dwell volume, extracolumn dispersion, and mixing efficiency as measured by mobile-phase compositional accuracy. Additionally, the dwell volume and extracolumn dispersion were independently and systematically varied to evaluate the resulting impact on resolution for a small-molecule test mixture during gradient elution. To account for these interinstrument variations, dwell volume and wash-out volume method translation and adjustment techniques were evaluated.
To support the need to get products to market more quickly and under tighter cost constraints, increased externalization of manufacturing and analytical development has occurred within the pharmaceutical and biotechnology industries (1,2). As activities are shifted to external laboratories, the diversity of instruments and configurations dramatically increases as compared to when all activities occurred within the same internal laboratory that typically had one instrument model and configuration. This instrument diversity has led to the observation of increased chromatographic separation variation.
This trend in conjunction with the shift from high performance liquid chromatography (HPLC) to ultrahighâpressure liquid chromatography (UHPLC) platforms has driven the need to characterize the expected interinstrument variations (3). Although variations between HPLC instruments from different manufacturers or with different module configurations contribute to chromatographic differences, typically the impact is not significant because of the inherent total volume of the system and the efficiency of the columns (4–6). With the shift to higher efficiency columns, the instrumental variations have a significant impact on the chromatographic performance. For example, the dwell volume to void volume ratio (VD/VM) varies between 2.1 to 2.8 for a 100 mm × 3.0 mm, 3.5-µm dp column on a binary versus quaternary pump HPLC. While using a 50 mm × 2.1 mm, 1.7-µm column on a binary versus quaternary UHPLC, the ratio can vary between 1.8 and 3.8. This difference illustrates the increased relative impact of instrumental variations on UHPLC methods.
Recommendations in the literature suggest adjustment of dwell volume, column temperature, and washâout volume to produce equivalent chromatographic results on different instruments (5,7–10). While these suggestions are available, the amount of expected interinstrument variation and the tolerability of these variations have had limited discussion. Additionally, the success rate of using these method translation and adjustment techniques has had limited unbiased evaluation, and the regulatory implications and required method validation studies to allow method adjustments require consideration.
The goals of this research are to understand the impact of instrumental parameters on the retention and resolution of analytes and better identify the cause of observed chromatographic differences between instruments for gradient separations. Additionally, method translation and adjustment techniques are evaluated with the goal of developing a framework to build quality into UHPLC methods to ease the method transfer process.
Experimental
All studies were performed using Waters H-Class instruments or Agilent 1290 instruments (binary and quaternary). All mobile phases were prepared using HPLCâgrade solvents. The gradient test mixture was purchased and used as is (Waters gradient test mix as part of the Acquity UPLC Absorb Start-up solution). The gradient test mix was selected to represent a simple small-molecule mixture for which retention would span the typical range of a pharmaceutical method. The gradient test mix method used a 50 mm × 2.1 mm, 1.7âµm Waters Acquity BEH C18 column at 40 °C. The mobile phase, multistep gradient, and flow rate were as follows: mobile-phase A: water; mobile-phase B: acetonitrile; 0–0.25 min, 10% B; 0.25–2.5 min, 10–95% B; 2.5–2.6 min, 95% B; 2.6–3.0 min, 95–10% B; 3.0–5.0 min, 10% B; 0.6 mL/min. The pharmaceutical sample used was a small-molecule peak identification solution, which contained molecules varying in acidity or basicity and hydrophobicity. This sample was selected because of its complex nature and sensitivity to variations in method conditions.
For all experiments, a 50 mm × 2.1 mm, 1.7-µm column was used. This column was selected based on the majority of the methods that are used within our laboratory. Compared to other typical column dimensions used for UHPLC methods (excluding 1.0âmm i.d. columns), these column dimensions represent a worst-case scenario in terms of impact from instrumental parameters.
Dwell Volume: The interinstrument variation in dwell volume was assessed by using a gradient of water and 0.1% acetone in water (11). The gradient was a 0–100% B linear ramp over 10 min. The column was replaced with a 1000 cm × 0.018 cm i.d. piece of polyetheretherketone (PEEK) tubing and was accounted for (248 µL) in determining the dwell volume of the system. The intersection of the isocratic (zero slope) and gradient slope of the chromatogram was used as the dwell time. For each UHPLC system, the dwell time was determined in triplicate and the average dwell time was used for the determination of the dwell volume. The dwell volume was calculated as shown in equation 1, based on the measured dwell time.

[1]
To evaluate the impact of dwell volume on the retention time and resolution of the analytes in the gradient test mix, the dwell volume was physically modified by adding different lengths of 0.17-mm i.d stainless steel tubing between the pump and the injector. Under the method conditions stated above, the gradient test mix was injected in triplicate and the average retention time of each analyte and the average resolution for each pair was determined. The average change in resolution was plotted as a function of the total dwell volume of the system (system dwell volume and volume of tubing added) for each analyte pair. The average change in resolution as a function of the change in dwell volume was calculated based on the experimental data.
Extracolumn Dispersion: The column was replaced with a zero-dead-volume union, 50:50 water–acetonitrile was used as the mobile-phase, and 1 mL of 0.1% acetone in water was injected. The extracolumn dispersion was determined by measuring the 4σ peak width of the acetone peak (12). For one UHPLC system, the extracolumn dispersion (ECD) was measured at different flow rates between 0.4–1.0 mL/min. The variation in ECD as a function of flow rate was found to be less than 1 µL. Therefore, only one flow rate was used for the measurement of ECD on the remaining instruments. For each UHPLC system, the measurement was determined in triplicate at 1.0 mL/min and the average ECD was calculated.
The effect of both precolumn and postcolumn ECD on the resolution of each analyte pair in the gradient test mix was evaluated. A known length of 0.12-mm i.d. PEEK tubing was placed between the injector and the column inlet to assess precolumn ECD and between the column outlet and the detector for postcolumn ECD. The same gradient test mix method conditions listed above were used for these experiments. The change in resolution for each analyte pair was plotted as a function of ECD added to the system. Based on this, the average change in resolution as a function of change in ECD was calculated for precolumn and postcolumn ECD.
Mixing Efficiency: For several instruments with different mixing volume and mixer type, the column was replaced with a zeroâdead-volume union and a step gradient of 0.1% acetone in mobile-phase B was generated as described by Medvedovici and David (13). The following mobileâphase combinations were assessed: water–methanol,
water–acetonitrile, and water–isopropanol. In all cases, %B was increased in increments of 10% and held at each level for 10 min. After the pump was delivering 100% mobileâphase B, the step gradient decreased in increments of 10% B until the pump was generating 0% mobile-phase B. The deviation from the theoretical gradient set point was determined by taking the difference between the instrumental set point and the %Bplateau:
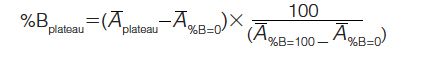
[2]
The average absorbance at the chromatographic plateau, A–plateau, was corrected for the absorbance at 0% B, A–%B=0 and normalized against the difference between the absorbance at 100% B, A–%B=100 and the absorbance at 0% B. For each UHPLC system–mixer configuration and mobile-phase combination, the average percent deviation was calculated. The measurement of the mobileâphase compositional accuracy as determined above was deemed suitable to assess the mixing efficiency since larger variations were observed for different mixer types than when measuring baseline noise.
Method Translation: For method translation and adjustment by dwell volume, the initial isocratic hold time was increased when moving from a system with larger dwell volume to a system with smaller dwell volume. The required isocratic hold time was determined by calculating the interinstrument difference in dwell volume and dividing by the flow rate to produce the time required to generate the required dwell volume. When moving from a system with a smaller dwell volume to a system with a larger
dwell volume, an injection delay was added to the
method. Within the software, the dwell volume difference was entered and the required injection delay was automatically calculated based on the method flow rate. The gradient test mix and the pharmaceutical sample were assessed for retention time and resolution consistency between the two instruments under evaluation. Moving from a system with a smaller dwell volume to a system with a larger dwell volume and vice versa was evaluated for each sample.
In addition to adjusting for dwell volume, the wash-out volume was also used as a method adjustment technique. A gradient of water and 0.1% acetone in acetonitrile from 0% to 100% B was generated with an additional isocratic hold at 100% B. The time required to transition from the gradientâslope region to the zero-slope region was used as the wash-out time for the system (7). The wash-out time and the flow rate were used to calculate the wash-out volume for each UHPLC system. The ratio of the washâout volume for the two instruments of interest was used to scale every step in the gradient programme. The gradient test mix was used to evaluate the consistency of retention time and resolution when adjusting for dwell volume and washâout ratio simultaneously.
Results and Discussion
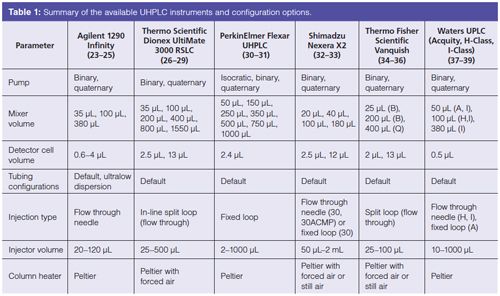
Instrument configuration variations can arise from differing pump type, mixer volume, detector cell volume, and tubing configuration - to name a few. When taken in combination, the potential for interinstrumental differences becomes quite high. Table 1 lists the common configurations available for several UHPLC systems currently on the market.
Dwell Volume: Dwell volume differences arise from instrumental parameters such as the pump configuration (binary or quaternary), the presence or absence of an in-line filter, mixer volume, and tubing configuration. The resulting dwell volume difference can affect the retention, selectivity, and resolution (14). The dwell volume and flow rate dictate the length of time required for the gradient to reach the column inlet, which affects the initial isocratic hold time experienced by the column and the speed of gradient change. Although dwell volume affects analytes throughout the entire separation space, variation in the dwell volume typically has a greater impact on weakly retained analytes; the elution of weakly retained analytes can vary from isocratic elution to gradient elution depending on the dwell volume of the system and the retention time of the analyte (9). Dwell volume can impact analytes throughout the retention window because of the resulting gradient shape dictated by the dwell volume to void volume ratio (10).
The dwell volume was measured for the UHPLC instruments within our laboratories to determine the amount of interinstrument variation that can be expected. The dwell volume for the instruments within our laboratories varied between 140 µL and 560 µL. For binary instruments, the dwell volume ranged from 140 µL to 220 µL and between 380 µL to 560 µL for quaternary instruments, depending on the configuration. To put this in perspective, for a method flow rate of 0.6 mL/min, the initial isocratic hold time would vary between 0.2 min and 0.9 min depending on the dwell volume of the system. Therefore, an analyte eluted between 0.2 and 0.9 min could be eluted under either isocratic or gradient conditions depending on the system used. Based on these potential variations, differences would be expected in the chromatographic separation between UHPLC instruments.
To evaluate the extent to which dwell volume affects analytes throughout the retention window, the dwell volume was physically modified by adding tubing between the pump and the injector. The resolution of the analyte pairs in the gradient test mix was measured as a function of system dwell volume (Figure 1). A change in resolution of 0.5 was chosen as an acceptable amount of variation. At this level of resolution variation, a dwell volume change up to 30 µL should not require method translation and adjustment. However, if the separation of interest cannot tolerate a resolution change of 0.5 between the critical pair, the amount of dwell volume variation acceptable would decrease as well.
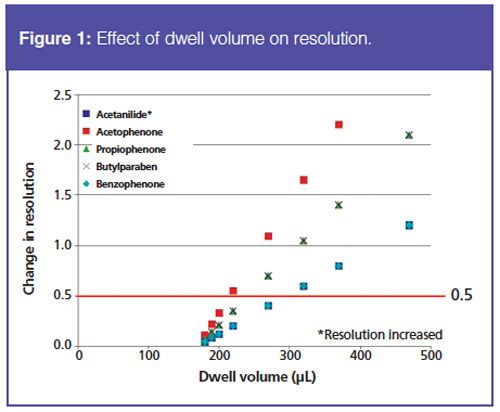
As expected, instruments with the same configuration and from the same manufacturer typically will not require method adjustment because of dwell volume. However, variations in tubing internal diameter between the pump and injector can produce a dwell volume difference that would require method translation and adjustment to maintain the chromatographic separation. Based on these findings, it is recommended to measure the dwell volume of the UHPLC system in which the method will be run and define the dwell volume used during method development within the analytical method.
Extracolumn Dispersion: In addition to dwell volume, the extracolumn dispersion can be found to vary between instruments producing differences in peak variance. The length and internal diameter of the connecting tubing and detector cell volume contribute to the ECD of the system (15). Although ECD contributes to band broadening in HPLC separations, as the variance related to the column decreases - as is the case with most UHPLC methods - the variance contributions from the system have an increased impact on the total peak broadening (14):
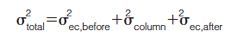
[3]
Although a large amount of research has addressed the effect of ECD on isocratic separations, few studies have addressed the impact on gradient separations. It is expected that the impact of the ECD will be less for gradient separations than for isocratic separations because of the gradient focusing that occurs, but this focusing cannot completely negate the contributions from the extracolumn dispersion (4,14).
The extracolumn dispersion for the UHPLC instruments within our laboratories varied between 12 µL and 50 µL. The majority of the instruments were found to have an ECD of 12–19 µL. A couple of instruments were found to have an ECD of approximately 50 µL, which resulted from tubing modifications that were made after installation. This excessive ECD can be easily remedied by changing the tubing, but the variability in ECD should be expected when transferring methods to external laboratories that may have many different system configurations.
Because the ECD was found to vary between the UHPLC instruments measured, the effect of ECD changes on resolution was evaluated. For both precolumn and postcolumn ECD, the change in resolution for the analyte pairs in the gradient test mix was measured as a function of added ECD (Figure 2). As expected, the change in resolution caused by additional precolumn ECD was less than the resolution change with added postcolumn ECD. Although the resolution change was more significant for postcolumn volume, the gradient focusing effect did not completely negate the effects of the precolumn ECD. Again, assuming that a resolution change of 0.5 is acceptable, changes of 10 µL and 4 µL are acceptable for the precolumn and postcolumn ECD, respectively. Therefore, based on the typical variation in ECD (7 µL) for the instruments measured, method translation and adjustment would not be required.
Comparing the effect of dwell volume and ECD on changes in resolution, the resolution change per volume change in ECD is more significant than that for dwell volume changes. However, the magnitude of interinstrument dwell volume variation is much greater than the ECD. Therefore, the dwell volume will have a more significant effect than the ECD on the separation.
Mobile-Phase Mixing: Historically, the mixing efficiency of high-pressure mixing (binary) instruments and lowâpressure mixing (quaternary) instruments has been debated. Typically, greater mixing volumes or complex mixers are put in place to compensate for the mixing inefficiencies of high-pressure mixing instruments for solvents of differing viscosity (16). However, high-pressure mixing is more suitable if outgassing occurs during mixing (17). As technology has improved, mixing inefficiencies have decreased. In practice, concerns are still present, particularly in cases when mixing volume is decreased to accommodate fast separations.
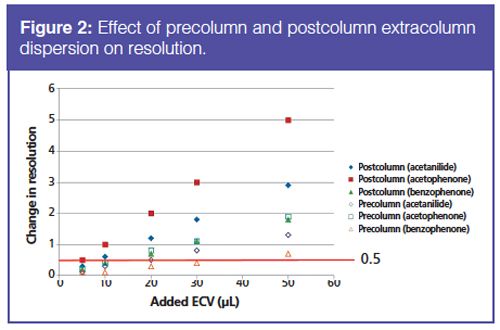
The mobile-phase mixing efficiency was assessed by measuring the %B deviation from the theoretical set point (Figure 3). For binary and quaternary systems with the same mixing volume (100 µL), the %B deviation was similar and without a trend. Furthermore, in most cases similar mixing efficiency was achieved for a high-pressure mixing system with a 35-µL mixer and a 100-µL mixer on a low- or highâpressure mixing system. When mixing water and methanol, the 35-µL mixer on the high-pressure mixing system was not sufficient to give adequate mixing as measured from compositional accuracy. In cases when water and methanol will be used, the mixing volume for a binary system should be at least 100 µL. For separations on the 5-min time scale, the additional dwell volume due to the 100-µL mixer as compared to the 35-µL mixer does not hinder separation speed, but can ensure sufficient mixing. Very fast separations (1–2 min) can use the smaller mixing volumes, but they may lead to mixing inconsistencies in the case of water and methanol mixing.
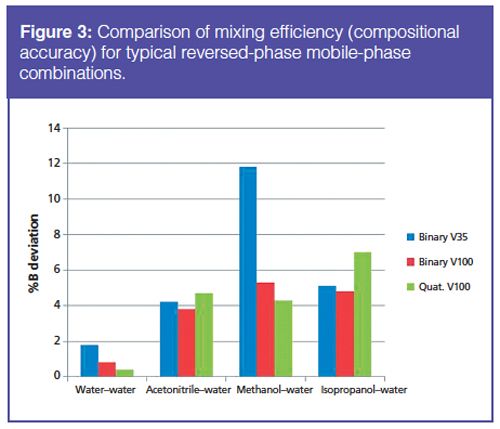
Method Translation Techniques: Running the gradient test mix method on instruments with differing configurations was found to produce significantly different chromatography (Figure 4). Because of this variation, method translation and adjustment is required. As previously discussed, several method translation techniques have been suggested in the literature and by instrument manufacturers (5,7–10). One approach is to adjust the isocratic hold time or injection delay to account for the interinstrument differences in dwell volume. Using the gradient test mix and pharmaceutical samples, this method was found to produce equivalent chromatographic retention. Although the retention was comparable, in some cases the critical pair resolution was inconsistent (Figure 5). This resolution variation may be due to other system differences such as actual column temperature, column axial temperature gradient, and extracolumn dispersion, which are not accounted for by adjusting the dwell volume. For highly complex methods and methods that are highly sensitive to method conditions, the ability to translate or adjust the method by simple techniques becomes more difficult.
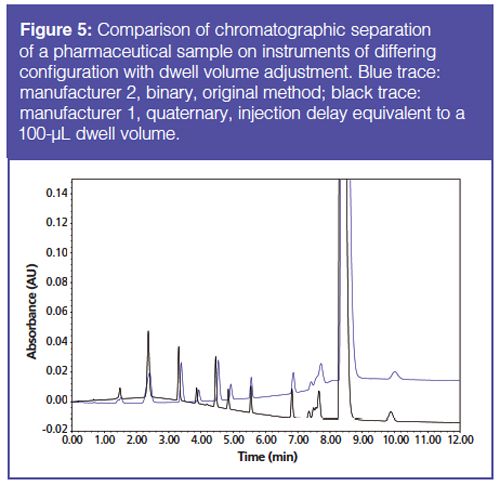
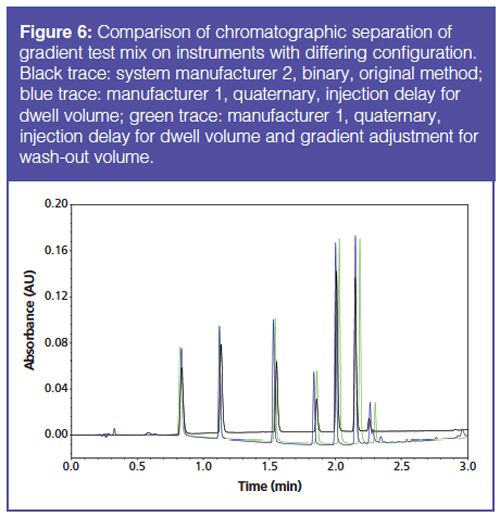
In addition to the dwell volume, analysts may need to account for the wash-out volume of the system. This volume is related to the mixer and manifests as a difference in the volume (time) over which the gradient changes (7). The difference results in an S-shaped transition or a Z-shaped transition depending on the washâout volume. Upon determination of the wash-out volume ratio between the instruments of interests, the method was modified to account for the dwell volume differences and the washâout volume ratio. Using the gradient test mix to assess this method translation technique, it was found that the interinstrument retention time deviation was greater than when using dwell volume alone (Figure 6). The wash-out volume ratio could be modified to align the chromatographic retention times on the two instruments, but this approach deviates from first principles of method translation and should be considered method development.
Based on the comparison of these method translation and adjustment techniques, the modification of the method based on dwell-volume differences has proven to be useful for the separations evaluated. To allow for dwellâvolume adjustments in the method, an initial isocratic hold should be included in the method that can be adjusted to accommodate instruments with differing dwell volumes. Additionally, the dwell volume of the method development system should be included in the method to allow for accurate adjustments. This method does not account for thermal differences, however, and they must be considered during the method validation studies if they are expected to affect the chromatographic separation.
Regulatory Considerations
Although method translation and adjustment can help ensure consistency of the chromatographic separation across differing UHPLC configurations, the method and modifications still need to comply with regulatory guidelines. According to United States Pharmacopeia (USP) <621>, the method can be adjusted for dwell volume and column temperature (±10 °C) (18). Although these modifications are acceptable, the adjustments should still be covered by the method validation robustness studies to allow for translation of the method during method implementation. Additionally, a method equivalency focus group as part of the IQ Consortium suggested that method modifications, including dwell volume, should be included in method validation robustness studies to allow for adjustments between instruments (19).
Additional method translation techniques have been developed by instrument manufacturers to compensate for instrumental differences and are promoted as not requiring additional robustness studies, but to date the acceptability by regulatory agencies is unknown (20–22). Although these translation techniques are based on first principles, their use without method validation robustness study coverage has yet to be accepted by the regulatory agencies. This lack of acceptance is not specifically because of the agencies disagreeing about the principles, but because of the hesitation of companies to submit methods that use this technology.
To stay within the regulatory guidelines and also allow method flexibility, the method should be evaluated over the entire range of expected dwell volumes. Within the method robustness studies, physically alter the dwell volume of the system and adjust the initial method isocratic hold time to maintain a constant isocratic step. Also, if a method is sensitive to the column temperature, assess the method at varying temperatures and include a temperature range within the method to allow for modifications to maintain the chromatographic performance specified within your system suitability criteria. Although most often the extracolumn dispersion should not vary by more than is tolerated by a typical UHPLC gradient separation, knowing the different system configurations that the method will be run on will allow the method developer to assess the potential impact.
Conclusions
The variation in dwell volume, extracolumn dispersion, and mixing efficiency were evaluated for the Waters H-Class and Agilent 1290 instruments present in our laboratories. For these method conditions, the greatest interinstrument variation was found to be caused by the dwell volume. Variations in ECD and mixing efficiency were observed, but at the expected level of variation neither is expected to significantly affect gradient separations. A dwellâvolume variation of less than 30 µL, a postcolumn ECD variation less than 4 µL, and a precolumn ECD variation less than 10 µL are not expected to require method translation or adjustment for the majority of gradient separations. Because the impact of these instrumental variations is dependent on the specific separation, these generalizations should be confirmed for the specific method.
In this study, the dwell volume and wash-out volume ratio method translation and adjustment methods were evaluated. The method found to be most useful for the methods evaluated was adjustment of dwell volume. To accommodate the need to adjust dwell volume, adding an initial isocratic hold in the method can ease the method translation–adjustment process. A further alternative is to develop methods on a column with a more modest column volume (that is, increased column internal diameter) to reduce the impact from instrumental parameters. Because of varying method complexity and analyte sensitivity to instrumental parameters, the specific method required for adjustment is expected to vary. To allow these types of method adjustments, the expected variations should be evaluated during the method validation robustness studies. The advantages in increased efficiency and shorter analysis time for UHPLC methods with columns of small volume and particle size are evident, but the time benefits of this technology have not been fully realized in a regulated environment because of the additional time required to assess method robustness. This missed opportunity points to the balance required between efficiency gains and increased impact from instrumental variations when using smaller column volumes.
References
- T.C. Kupiec, Contract Pharma, July/August www.contractpharma.com/issues/2003-03/view-features/analytical-outsourcing (2005).
- R. Rodriguez-Diaz, K. Bullock, S. Durban, and T. Sullivan, Pharm. Outsourcing, 11 http://www.pharmoutsourcing.com/Featured-Articles/37656-Common-Practices-for-Analytical-Methods-Transfer/ (2010).
- M.W. Dong, LCGC North Am.25(7), 656–666 (2007).
- R.E. Majors, LCGC North Am.32(11), 840–853 (2014).
- D. Guillarme, J.-L. Veuthey, white paper, Guidelines for the use of UHPLC Instruments, (Laboratory of Analytical Pharmaceutical Chemistry [LCAP], 2008) http://www.unige.ch/sciences/pharm/fanal/lcap/Guidelines%20for%20the%20use%20of%20UHPLC%20instruments%20-%20site%20WEB%20labo.pdf.
- H.K. Brandes, D.T. Nowlan III, J.W. Best, and R.A. Henry, LCGC Europe26(s11) 20–27 (2013).
- T. Taylor, LCGC North Am. 32(6), 450 (2014).
- J.W. Dolan, LCGC Europe26(6), 330–336 (2013).
- D. Guillarme, D.T.T. Nguyen, S. Rudaz, and J.-L. Veuthey, Eu. J. Phar. Biopharm.68, 430–440 (2008).
- P. Petersson, M.R. Euerby, and M.A. James, LCGC Europe28(6), 310–320 (2015).
- J.W. Dolan, LCGC North Am. 24(6), 458–466 (2006).
- Mac-mod technical report, How to Measure and Reduce HPLC Equipment Extra-column Volume, http://www.mac-mod.com/pdf/technical-report/MMA084-ReduceECV.pdf.
- A. Medvedovici and V. David, Encyclopedia of Chromatography, 3rd ed. (Taylor and Francis, Oxford, UK, 2010), pp. 1947–1962.
- S. Fekete, I. Kohler, S. Rudaz, and D. Guillarme, J. Pharm. Biomed. Anal.87, 105–119 (2014).
- S. Fekete and J. Fekete, J. Chrom. A1218, 5286–5291 (2011).
- J.W. Dolan and L.R. Snyder, Troubleshooting LC Systems: A Comprehensive Approach to Troubleshooting LC Equipment and Separations, 1st ed. (Springer Science + Business Media, New York, New York, USA, 1989), pp. 180–200.
- M.W. Dong, Modern HPLC for Practicing Scientists, 1st ed. (John Wiley and Sons, Hoboken, New Jersey, USA, 2006), p. 82.
- USP <621> Chromatography, USP-NF 38 (The United States Pharmacopeia Convention, 2015).
- F. Diana, J. Hofer, M. Chatfield, Y. Huang, A.M. Piess, R. Burdick, Q. Wang, M. Argentine, Z. Williams, H. Fang, H. AbdulâKader, S. Karmarkar, L.M. Nair, N. Benz, and T. Natishan, PharmTech38(10), (2014).
- A.G. Huesgen, Agilent Technology Overview, 5990-9113EN (2011).
- K.L Fountain, Waters Application Note, 720004129 (2013).
- P. Hong and P.R. McConville, Waters Application Note, 720005332EN (2015).
- Agilent 1290 Infinity LC System Manual and Quick Reference Guide, G4220-90301 (Agilent Technologies, 2012).
- Agilent 1290 Infinity Diode Array Detector, 5990-6107EN (Agilent Technologies, 2013).
- Agilent 1290 Infinity Autosampler, 5990-6123EN (Agilent Technologies, 2010).
- Thermo Scientific Dionex UltiMate 3000 Thermostatted Column Compartment, PS70474_E (Thermo Fisher Scientific, 2013).
- Thermo Scientific Dionex UltiMate 3000 Series Diode Array Detectors Operating Instructions, 4820.8250 (Thermo Fisher Scientific, 2013).
- Thermo Scientific Dionex UltiMate 3000 Pumps, PS70108_E (Thermo Fisher Scientific, 2013).
- Thermo Scientific Dionex UltiMate 3000 Series WPS-3000 Autosamplers Operating Instructions, 4828.2250 (Thermo Fisher Scientific, 2013).
- S. White, PerkinElmer Chromatography Sample Specialist, e-mail communication.
- Mixer Volume Selection Grid, Flexar LC PDL V 2011-1 for Chromera and TotalChrom, version 2010-2 (2010).
- C. Jester, Shimadzu Sales Engineer, e-mail communication.
- Nexera XR Specification, C196-E083A (PerkinElmer, 2015).
- D. Deliman, Thermo Scientific Technical Sales Representative, e-mail communication.
- M. Heidorn, S. Fabel, and F. Steiner, “Thermostatting in UHPLC: Forced-Air Mode, Still-Air Mode, and Method Transfer,” Scientific Poster Note, PN71314-ISC-EN 0914S (Thermo Fisher Scientific, 2014), https://tools.thermofisher.com/content/sfs/posters/PN-71314-ISC2014-UHPLC-Thermostatting-PN71314-EN.pdf?elqTrackId=263150ecb7474e3ab29cc9112acc137f&elqaid=1619&elqat=2.
- Thermo Scientific Vanquish – Binary Pump H, PS71186-EN 0714M (Thermo Fisher Scientific, 2014).
- Acquity UPLC System Operators Guide, 71500082502, Rev. C (Waters Corp., 2006).
- Acquity UPLC H-Class System Guide, Rev. A (Waters, 2010), www.waters.com/webassests/cms/support/docs/acquity_uplc_h_class_system_guide.pdf.
- Acquity UPLC I-Class System (SM-FTN) Instrument Specifications, 720003948EN (Waters Corp., 2013).
Laura E. Blue, Tawnya Flick, and David Semin work at Amgen Inc., in Thousand Oaks, California, USA.

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.









