How Complete Are Your Chromatographic Data?
This article will explore what is meant by "complete data" in the context of a chromatography data system and, with an analysis of FDA 483 citations, explore the problems when a laboratory fails to understand this phrase.
US Good Manufacturing Practice (GMP) regulations for quality control (QC) laboratories require that laboratory records shall include complete data derived from all tests. We will explore what is meant by "complete data" in the context of a chromatography data system and, with an analysis of the United States Food and Drug Administration (FDA) 483 citations, explore the problems when a laboratory fails to understand this phrase. We will also consider if complete data includes the electronic files generated during the course of a chromatographic analysis or just the signed paper printouts. This is the first in a two-part column and in the second part we will look at paper versus electronic records in the context of complete data, what happens when things go wrong, and what is the European approach.
Reviewing the United States Food and Drug Administration (FDA) compliance statistics for regulated laboratories is an interesting, but somewhat sad, way to spend time. However, as it's raining heavily today and for the foreseeable future, the two options available to me are either build an ark or write this column. As I'm not the best handyman in the world, the writing won hands down.
The title of this column is derived from the section of the FDA's Good Manufacturing Practice (GMP) regulations for finished pharmaceutical products specifically concerned with laboratory records — 21 CFR 211.194(a) which states:
Laboratory records shall include complete data derived from all tests necessary to assure compliance with established specifications and standards, including examinations and assays as follows: (1)
Judging from current FDA 483 citations from 2006 to 2012 which I will discuss now, many regulated quality control (QC) laboratories have problems providing complete data during an inspection which is a major issue.
Annual FDA 483 Citation Reports
When the FDA pop in for tea, biscuits, and a cosy chat, there might be the odd occasion when they notice a non-compliance with the way that a laboratory operates (this is never your laboratory is it?). Every non-compliance that has not been resolved before the conclusion of the audit will be documented on FDA Form 483 which is handed to the head of the site at the end of the inspection. Copies of these forms are collated by the agency and published each FDA fiscal year. On the FDA website there is a page entitled Inspections, Compliance, Enforcement, and Criminal Investigations (2) and I would like to thank Paul Smith of Agilent Technologies for finding this web page and sharing it with me.
The page is managed by the Office of Regulatory Affairs (ORA) and provides information about the 483 observations generated across the areas that the FDA regulates, for example, drugs and food medical devices. If a 483 observation form is prepared by the FDA software application called TurboEIR, any citations generated are included in the annual spreadsheets available for download at the foot of that web page. There are currently spreadsheets for each FDA fiscal year starting from 2006 to 2012 inclusive. However, be prepared:
- Each spreadsheet contains citations for all areas that the FDA regulates, so you need to carefully review to find the tab for the area you are interested in, such as medical devices or pharmaceutical. This way you avoid having to wade through citations dealing with food, although the Capitol Cake Company or Indian Foods and Spices warning letters (3, 4) can be a diverting and surprising source of entertainment, provided, of course, that you are not a customer of these establishments.
- When you open the tab dealing with pharmaceutical companies, the citations need to be sorted to find the area of the GMP regulations you are interested in.
- These spreadsheets are not a comprehensive listing of all inspectional observations because only 483s prepared using TurboEIR are included in the listings; therefore data are not comprehensive but give a representative "snapshot" of compliance, or rather non-compliance, in the pharmaceutical industry, and in quality control laboratories in particular.
The area of the GMP that I am interested in is the section that deals with laboratory records or §211.194. §211.194 is divided into five clauses covering the following topics:
- a. Testing records
- b. Test method modification records
- c. Reagents & standards testing records
- d. Laboratory equipment calibration records
- e. Stability testing records
The phrase "complete data" applies to §211.194(a) and "complete records" is a consistent requirement for the remaining clauses of §211.194. I consider the two phrases to be equivalent and to mean the same thing. Although the title of this column is complete data, the principles that we discuss here also apply to the other sections of this regulation.
I have searched the seven fiscal years of 483 observations from the downloaded spreadsheets (5–11) for the number of citations against the clauses in §211.194 and these are presented in Table 1. There was a total of 878 citations against the clauses of §211.194 over the seven years, with the number of citations for non-compliances in any one year ranging from 104 to 145. However, the distribution of the non-compliances for each clause was more interesting.
- The area where there were the most problems was §211.194(a) testing records which constitutes 80% of the non-compliances.
- The other areas with non-compliances are sections §211.194(c), reagents and standards testing records, and §211.194(d), laboratory equipment calibration records. (To some extent this will be linked to analytical instrument qualification which will be found under §211.63 but any non-compliances under §211.63 are not included in Table 1).
- Verifying analytical procedures under actual conditions of use and stability testing records had far fewer citations — in the order of ~2% each.

Table 1: Number of all FDA 483 citations for §211.194 non-compliances 2006â2012 (5â11).
It is not surprising that §211.194(a) has the highest number of citations as the main focus area for an inspection will be the analysis of batch records for product release.
A further analysis of the data within the various sub-clauses of §211.194(a) shows that three of the major causes of regulatory citations in this area are:
- Failure to identify the test method used adequately;
- Failure to record sample weights taken during the analysis;
- Failure to have the initials of signature of the reviewer.
In essence, these citations are a fundamental failure of either the tester or the reviewer to do their respective jobs correctly, and contrast with the FDA requirements for laboratory "data integrity" as documented in the scope of the laboratory audit (objective 3) of the Compliance Policy Guide 7346.832 on pre-approval inspections (12).
In this column, I will focus on looking at complete data as it relates to testing raw materials, in process samples and finished products. During the course of the discussion, we will also look at the other sections of §211.194 impact complete records for reagents and standards used in our testing as well as laboratory equipment and calibration records. I will then look briefly at the corresponding European Union GMP regulations for laboratory records.
Laboratory Testing Record Requirements
We have seen that the worst area of compliance with the GMP regulations in the regulated laboratory is §211.194(a). This is not surprising because release testing is the major function of regulated QC laboratories. However, we need to see what the detailed requirements are for this section. You will recall that earlier I quoted the beginning of §211.194(a) when explaining the title of this "Questions of Quality" column. A list of eight detailed requirements of the analytical data acquired during the course of analysing a sample then follows, as shown in Table 2, which includes the initials or signature of the person conducting the test. Also required are the initials or signature of a second person checking the analysis to see that data have been acquired correctly, calculations have been performed correctly, and applicable procedures have been followed. This is the classic "four eyes principle" that we discussed in an earlier "Questions of Quality" column (13). However, let us remain on track to review the phrase "complete data". In Table 2 I have highlighted the key items for each of the eight requirements of §211.194(a). Please note, as mentioned earlier, there are other sections of §211.194 that also ask for complete data of standardization of reagents and standards used for tests and calibration of our instruments amongst others, so you are advised to read the whole of this regulation to see the scope of what is meant by "complete data". For the purposes of this discussion, we are focusing on the complete data with respect to sample analysis.

Table 2: GMP regulations for laboratory data (1).
Complete Data
You would think that two simple words could not cause a large amount of confusion in regulated laboratories. From a handy dictionary available in my home (14) we can obtain the following definitions:
- Complete: having every necessary part or entire;
- Data: a series of observations, measurements, or facts.
Therefore my interpretation of complete data from the regulation and the definitions above is the entire series of observations or measurements for a single test or chromatographic run. What complete data does not mean is that the analyst makes a selection of the best data that fits his or her testing requirements. This is where subsection 211.194(a) (4) is very explicit:
A complete record of all data secured in the course of each test, including all graphs, charts, and spectra from laboratory instrumentation, properly identified to show the specific component ......, or drug product, and lot tested.
To summarize, everything that you do — from setting up the chromatograph, to the test itself and any further testing — is covered by the regulation above. You cannot omit anything. You cannot delete anything. So, what does this mean in practice?
The Analytical Process
Let us focus for a few minutes on how we conduct chromatographic analysis in an analytical laboratory. Samples are taken, stored, and prepared according to the appropriate sampling and analytical procedures and the end result is typically the samples, standards, blanks, and any quality control standards are ready in vials for analysis. This analytical process is shown in Figure 1. In each stage of the process, records will be generated that are essential for compliance with GMP regulations. However, these laboratory records are also good analytical science and should be instilled in all analytical scientists early in their education and training.
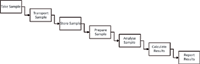
Figure 1: A typical laboratory analytical process.
After the sample has been taken either by warehouse or production staff, or a member of the QC laboratory, it is transported to the laboratory for analysis. Samples may be stored in predefined conditions until there are sufficient samples to make a batch for analysis or a single sample can be analysed. In the process defined in Figure 1. This may be a sample preparation stage which may be as simple as putting a weighed sample aliquot into a volumetric flask and dissolving it, or it may involve a more complex sample preparation scheme before the sample is ready for presentation to the chromatograph.
As the samples are prepared for analysis the analyst will document details about the sample information. This can be done in laboratory notebooks, controlled pro-forma worksheets, or using a laboratory information management system (LIMS) or electronic laboratory notebook. This information will include batch or lot number, sample identities, sample weights taken, preparation details, and dilutions used. In addition, the identities of reference standards used and their preparation, the sample analysis sequence and identification of the chromatograph used in the test are also documented. Although all of this is required by regulation, it is nonetheless good analytical science to ensure that all of this data is collected and retained for review (see the items listed in Table 2). Documentation of analytical work in this way is an analytical expectation, not an exception, regardless of working in a regulated or unregulated laboratory.
Now we need to prepare the chromatograph for the analysis, turn the instrument on, and, if appropriate, let it warm it up and equilibrate. What happens next depends on how the laboratory has documented its analytical procedures. Typically, there is a system suitability test or point-of-use check performed to ensure that the chromatograph is working within acceptable limits before committing to the analysis of samples.
Remember that we are "in the course" of a test, as noted in 211.194(a)(4) in Table 2, and that any data present or generated needs to be documented and retained. Therefore, even if you are just "evaluating" that the instrument works, the sample or samples used in this process need to be documented in your standard operating procedure (SOP) or analytical procedure and the data generated needs to be maintained.
Assuming that the reference injection produces acceptable results, the analysis proceeds per the documented analytical procedure:
- Samples are analysed using the selected chromatograph, the details of which are recorded in the instrument log book. Any data files generated by the chromatograph during the course of analysis must be uniquely identified and saved by the chromatography data system (CDS) because they are part of the complete data equation as per §211.194(a)(4) in Table 2.
- In addition, the method files, injection sequence files, processing algorithms, or methods must be documented and also linked to the data files generated and stored in the CDS.
- The question of paper or electronic records is discussed in part 2 of this column in much more detail, but suffice it to say that the electronic records are an essential part of complete data and must be saved regardless of the fact that paper copies of these records are printed out.
- If the analysis is qualitative, then no calculations will be performed on the data and perhaps a statement of identity confirmation is all that is required. However, if quantitative analysis is performed, validated calculations would be used which are typically either within the CDS or contained in a separate spreadsheet, in PDF form, or in additional software. Records of these calculations will be needed as part of complete data.
- Changes to data will be recorded in the audit trail of the CDS. Remember that these entries are a key component of complete data and are essential for ensuring the integrity of the data generated. The audit trail entries should be inspected if they involve changes made by the tester.
- Any problems with the analysis may require the samples to be reanalysed, but only in conformance with the laboratory procedure on out of specification (OOS) results. Again, all sample and standard chromatograms generated are required to comply with the complete data requirement.
- After the analyst has finished their portion of the work, it is reviewed and checked by a second individual to check that the work is scientifically sound, the appropriate procedures were followed, data have been calculated properly, electronic records and paper records match, and the audit trail of the data system has been reviewed as another means of ensuring data integrity.
- Finally, when all results are complete and have been checked, a certificate of analysis is generated for the sample and the batch or lot can be released if in specification.
This procedure might seem straightforward and simply stating the obvious; however, as we have seen earlier, there are a number of laboratories that, through either design or incompetence, fail to achieve even a basic level of analytical competence as outlined in this article. In the second part of this article we will look at paper vs. electronic records in the context of complete data, what happens when things go wrong, and what is the European approach to this area.
"Questions of Quality" editor Bob McDowall is Principal at McDowall Consulting, Bromley, Kent, UK. He is also a member of LCGC Europe's Editorial Advisory Board. Direct correspondence about this column should be addressed to "Questions of Quality", LCGC Europe, 4A Bridgegate Pavilion, Chester Business Park, Wrexham Road, Chester, CH4 9QH, UK, or e-mail the editor-in-chief, Alasdair Matheson, at amatheson@advanstar.com
References
(1) Current Good Manufacturing Practice for Finished Pharmaceutical Products, 21 CFR 211, (2009).
(2) Inspections, Compliance, Enforcement, and Criminal Investigations http://www.fda.gov/ICECI/EnforcementActions/ucm250720.htm
(3) FDA Warning Letter, Capitol Cake Company, August 2008.
(4) FDA Warning Letter, Indian Foods and Spices, August 2012.
(5) FY 2006 Inspectional Observation Summaries – download from reference 2.
(6) FY 2007 Inspectional Observation Summaries – download from reference 2.
(7) FY 2008 Inspectional Observation Summaries – download from reference 2.
(8) FY 2009 Inspectional Observation Summaries – download from reference 2.
(9) FY 2010 Inspectional Observation Summaries – download from reference 2.
(10) FY 2011 Inspectional Observation Summaries – download from reference 2.
(11) FY 2012 Inspectional Observation Summaries – download from reference 2.
(12) FDA Compliance Programme Guide 7346.832 Pre-Approval Inspections (published 2010 but effective from 2012).
(13) R.D. McDowall, LCGC Europe 24(4), 208–216 (2011).
(14) Collins Dictionary & Thesaurus (William Collins, London, UK, 1988).
(15) Electronic Records; Electronic Signatures final rule (21 CFR 11) (1997).
(16) R.D. McDowall, LCGC Europe 25(4), 194–200 (2012).
(17) Questions and Answers on Current Good Manufacturing Practices, Good Guidance Practices, Level 2 Guidance — Records and Reports. Part 3 (2010) http://www.fda.gov/Drugs/
(18) FDA Guidance for Industry, Part 11 Scope and Application, 2003.

Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.







