How Can LIMS Help Ensure Data Integrity?
In the world of data integrity, the focus is typically on the data and the numbers. How can technology, such as a laboratory information management system (LIMS), help to ensure data integrity?
R.D. McDowall, R.D. McDowall Ltd., Bromley, Kent, UK
In the world of data integrity, the focus is typically on the data and the numbers. How can technology, such as a laboratory information management system (LIMS), help to ensure data integrity?
The focus on data integrity has been on the culture, procedure, and training to ensure that the data generated is complete, consistent, and accurate. In this instalment of “Questions of Quality” we look at interfacing instruments to informatics solutions such as a laboratory information management system (LIMS) to help ensure data integrity and faster working practices.
And Then There Were Three
We have a saying in the UK, you can wait ages for a bus and then three come along at once. Well it is not quite the same in the regulated world with health authority timescales glacial at times. However, the regulatory equivalent is that in the space of just over a year we have had three data integrity guidance documents issued by three regulatory authorities:
- MHRA Data Integrity Guidance for industry, version 2 in March 2015 (1)
- WHO Draft Guidance on Good Data and Record Management Practices in September 2015 (2)
- FDA Draft Guidance for Industry on Data Integrity and cGMP Compliance in April 2016 (3)
The guidance documents have a little overlap and are very complementary in the advice that they offer to industry. One area of overlap, albeit small, is the comment tha data from analytical balances should either be recorded on attached printers or by direct data capture to an automated system (1,2).
Paper Versus Technology
This is an interesting situation. Firstly, regulatory authorities do not trust an individual to record a balance weight by observation. This is not unreasonable because an analytical balance weighing can have a farâreaching impact if it is wrong. For example, if a sample weight is wrong either a batch could be wrongly passed or failed, or if an analytical reference standard is incorrectly recorded then several batches or even a whole study could be incorrect. Hence, the need to see documented evidence of the actual weighing.
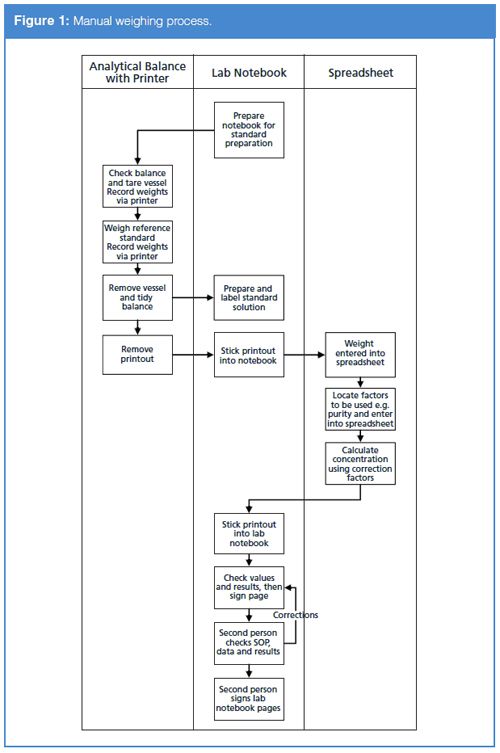
However, do you like drowning in paper? Think of the process you will have to endure (Yes, I have used the word endure deliberately). You will typically perform the following actions when weighing a sample of an analytical reference standard (the main actions are visualized in Figure 1):
Check that the balance is the correct one or type for the weighing you are going to perform.
Check that the balance is qualified and within calibration.
Write up the balance information in your laboratory notebook or on a controlled worksheet.
Perform a point-of-use check (typically with reference masses over the calibration range or using the internal calibration of the balance).
Write the information in the instrument logbook.
Take reference material and write the information into your laboratory notebook.
Just writing this make me breathless and we have not done any real work yet!
- Weigh a weighing vessel
- Tare the balance
- Weigh the reference standard
- Print the weighing sequence
- Complete the instrument log book for the weighing
- Stick the printout into your laboratory notebook or controlled worksheet
- Transfer the vessel to the volumetric flask
- Dissolve the material and make up to volume
- Label the flask with the solution identity, storage conditions, and expiry date, etc.
- Record the information in your laboratory notebook
Finished! Err, not quite - what are you going to do with the reference solution? It is not just going to sit in storage is it? No, we are going to use it so we will need to write the solution identity and other information into the analytical run documentation each time we use the solution. Please don’t tell me that you are going to do some calculations as well by entering data into a spreadsheet and printing out the result? Writing, more writing, and even more writing. And that is not all; think of the second person reviewer, transcription error checking, and more transcription error checking.
Let me ask you a very personal question. Are you lazy? The answer to this question should be an unequivocal “Yes”. The reason is that you don’t want to go through all of this trouble each time you weigh something. Be lazy and automate the process - even the regulators are with you on this! If a process is automated, validated, and transparent, then it becomes more inspection friendly.
One of the reasons for this is that even if you have a printer attached to a balance, the date and time stamp can be altered manually. However, when you are on-line, the date and time can be automatically applied to the data as it is transferred to an informatics solution on the network.
Data Acquisition at the Point of Origin
One of the key requirements for automation is to acquire data electronically at the point of origin. This means no printing of results, no writing down of information, and no manual transfer or retyping of data. The key to data integrity is to control the primary data acquisition stage so that the foundation of data correctness, accuracy, and completeness at the point of origin is assured. If you are unable to control these elements, the rest of any automated process is worthless.
Let us look at how we can automate the process using a LIMS in addition to some other informatics solutions. We need to interface the analytical balance to the LIMS. Most analytical balances can be used as a terminal so the LIMS can be operated from the balance screen. This eliminates two potential sources of data integrity error or falsification: access to the clock and not automating the whole acquisition process, which means that the overall procedure is enforced technically as can be seen in Figure 2.
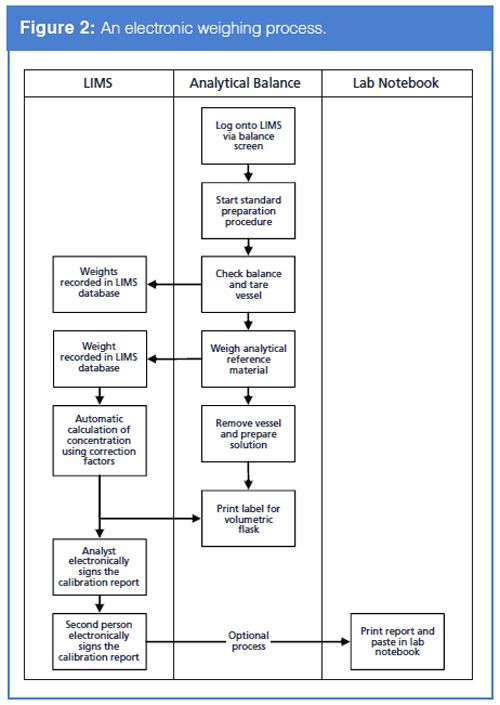
Some of the benefits of this approach are:
- It ensures that the correct type or, if required, an individual balance is selected for use and that it is qualified. Attempts to select a different or unqualified balance will be blocked by the LIMS.
- The calibration checks, if using external weights, can be captured by the LIMS to determine if the balance meets predetermined acceptance criteria. In addition, the cumulative calibration data can be plotted to see if there are any trends apparent over a user-defined time period.
- Time and date stamps will be applied to the data by the LIMS server, which is linked to the network time server, which itself is linked to a trusted time source external to the organization such as a national time source, GPS system, or a network time protocol (NTP) server.
- Typically, the analytical reference substance is entered into the LIMS database and has all the requisite information such as identity, lot number, acquisition date, purity, etc. The container will be labelled both in human readable text but also bar coded. If the balance has a bar code reader interfaced, then the identity of the standard can be verified as the weighing of the material begins.
- The whole of the balance weighing can be captured within the LIMS.
- Any calculations regarding purity or salt form to base conversion will be performed in the LIMS during the weighing, obviating the need to use a spreadsheet.
- The resulting reference solution can have a label printed by the LIMS and the use of this up to its expiry date can be tracked by the LIMS.
- The use of the reference standard material over time can also be seen and if required tracked easily.
Virtually the whole of the operation can be controlled and data captured by the LIMS, or indeed any suitable laboratory informatics application that is able to perform these tasks. There is little that needs to be recorded outside of the system.
Indeed, when you compare the two processes you wonder why chromatographers still work in the old-fashioned paper-based way. Just look at the simplicity of the electronic process in Figure 2 in comparison to the complexity of the manual process in Figure 1. This raises two questions: Are chromatographers masochists? Or is it just the sadists in quality assurance that perpetuate these inefficient working practices?
Extend the Principle: Eliminate Hybrid Systems
Working with hybrid systems - computerized systems that generate electronic records with signed paper printouts - is the WORST possible world to be in. The laboratory has to manage two incompatible media formats: paper and electronic records. The best advice is to eliminate these systems by using electronic systems. Obviously in LCGC Europe we will look at chromatography data systems (CDSs). In the past this column has discussed electronic working with a case study example (4), however, the focus was on process efficiencies within the CDS itself and there was little consideration of interfacing the system to a LIMS. Therefore in our discussion we need to extend the scope to consider how a LIMS interfaced to a CDS can help ensure data integrity.
What the Regulators Want
In section 9 of the WHO guidance under the attributable section there is an interesting discussion about
the hybrid systems (2) that is reproduced below. All I have done is to add bullet points, my comments underneath each one, and bold text to make it easier to read and understand.
A hybrid approach may be used to sign electronic records when the system lacks features for electronic signatures.
Regulators will not tell companies that hybrid systems are not suitable because laboratories can still use them, but read on.
- To execute a handwritten signature to an electronic record, a simple means to do so would be to create a singleâpage controlled form associated with the written procedures for system use and data review that would list the electronic dataset reviewed and any metadata subject to review, and would provide fields for the author, reviewer, and/or approver of the dataset to apply a handwritten signature.
This is different to the way most laboratories review data because the current emphasis is on the paper printouts, with little emphasis placed on the electronic records including relevant audit trail entries.
- This paper record with the hand-written signatures should then be securely and traceably linked to the electronic dataset, either through procedural means, such as use of detailed archives indexes, or technical means, such as embedding a certified true copy scanned image of the signature page into the electronic dataset.
What a nuisance this is - do you really want to work this way?
- The hybrid approach is likely to be more burdensome than a fully-electronic approach, therefore, utilizing electronic signatures, whenever available, is recommended.
Here’s the killer - get rid of hybrid systems because they waste time and effort.
LIMS Interfaced with a CDS
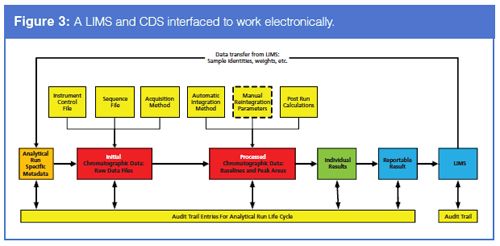
In light of the previous section, let us consider a CDS and LIMS interfaced with each other - how could this setup help our quest for data integrity in an analytical laboratory? Readers may recall a recent “Questions of Quality” column that Chris Burgess and I authored about records in a CDS (5). In this we presented a diagram of the various electronic records generated in a CDS. The figure is reproduced here in Figure 3 but it has been modified in the following important aspects:
- The LIMS is shown on the rightâhand side of the diagram.
- Assume that the analytical balance referred to at the start of this column is also interfaced to the LIMS and that sample weights can be linked to the correct sample identities in the database.
- Electronic transfer of data from the LIMS to the CDS to help set up the analytical run can include download of sample identities and sample weights used for the analysis and possibly reference standard purities and water content depending on the type of analysis performed.
- Dilutions made during the sample preparation phase of the analysis will probably need to be entered manually into the CDS sequence file as now.
- Transfer from the CDS to the LIMS can vary from laboratory to laboratory. This transfer can vary from all integrated peak areas per sample identity of all injections so that all subsequent calculations are performed in the LIMS (typically this is for bioanalytical LIMS) to the calculated reportable results that is compared with the specification in the LIMS. Regardless of the data content, the transfer must be electronic.
- If a file is transferred between the CDS and a LIMS, which is parsed to extract the requisite data, the file must be protected from tampering between transfer and parsing. Ideally such a mechanism would not be used - direct transfer between the two systems is much better.
Importance of the Second Person Review
One of the keys to ensuring data integrity is the second person review. The US GMP regulations for this can be found in 21 CFR 211.194(a)(8) (6) and states:
The initials or signature of a second person showing that the original records have been reviewed for accuracy, completeness, and compliance with established standards.
With the publication of the draft FDA guidance on data integrity (3) there are further requirements for the second person review to detect any attempt as falsification of data:
16. Should personnel be trained in detecting data integrity issues as part of a routine CGMP training program?
Yes. Training personnel to detect data integrity issues is consistent with the personnel requirements under § 211.25, which state that personnel must have the education, training, and experience, or any combination thereof, to perform their assigned duties.
Therefore, as part of the second person review, there needs to be more time spent checking to see if any data has been manipulated or falsified. This will slow down the data review, particularly with hybrid systems.
Another requirement is for the explicit review of audit trail information associated with analysis, which has two of the 18 questions dedicated to the topic:
7. How often should audit trails be reviewed?
FDA recommends that audit trails that capture changes to critical data be reviewed with each record and before final approval of the record. Audit trails subject to regular review should include, but are not limited to, the following: the change history of finished product test results, changes to sample run sequences, changes to sample identification, and changes to critical process parameters.
FDA recommends routine scheduled audit trail review based on the complexity of the system and its intended use.
In the past there was an implicit expectation for review of audit trail entries as noted with the Able Laboratories 483 in 2005 (7). However, the new guidance brings the FDA into alignment with EU GMP Annex 11 (8), which in clause 9 requires audit trails to be reviewed regularly and under clause 1 risk assessment needs to be applied as to the frequency of the review. Harmonization at last!
8. Who should review audit trails?
Audit trails are considered part of the associated records. Personnel responsible for record review under CGMP should review the audit trails that capture changes to critical data associated with the record as they review the rest of the record (for example, §§ 211.22(a), 211.101(c), 211.194(a)(8), and 212.20(d)). For example, all production and control records, which includes audit trails, must be reviewed and approved by the quality unit (§ 211.192). This is similar to the expectation that cross-outs on paper be assessed when reviewing data.
As can be seen the lucky individual who has picked up the poisoned chalice to review the audit trail entries is the second person reviewer (9). Let us explore what this means in practice with our LIMS and CDS system.
Second Person Review in Practice
We need to consider the main elements for the data and metadata review for a batch of samples. Before beginning this discussion, I will make the following assumptions:
1. The whole process is electronic and uses electronic signatures. Exactly where the signatures are applied is left to the individual implementations but there are two main approaches: the first is to sign in the CDS (performer of the test) and then in the LIMS (reviewer of the work), or alternatively just in the LIMS (both performer and tester).
2. Technical controls have been implemented and validated to ensure that the process is followed and enforced by the software applications. Configuration settings in both applications are set for protection of electronic records.
The scope of the second person review should cover the analytical process from sample storage to reportable result and is not confined to the boundaries of a specific system. However, as the focus of this column is how a LIMS can help data integrity we will look at the two systems shown in Figure 3 only. Therefore, for the purposes of this discussion, the second person review begins in the CDS, including the chromatograph used for the analysis, and ends in the LIMS. Reiterating my earlier point, the review covers the analytical process not individual applications. If the second person review was split into two phases based on the CDS and the LIMS, then there is a possibility that the transfer between the two systems is omitted.
Now we have to consider what the second person reviewer has to review: both the data and the associated metadata, as shown in Figure 3. The problem is that in a risk-based world how can we focus on what is really critical to meeting the requirements of both the FDA guidance (3) and EU GMP Annex 11 (8)?
Table 1 presents the main questions for consideration in the review along with a risk-based approach to focusing the second person review. The focus is mainly on the electronic records and is not, repeat not, a comprehensive approach.
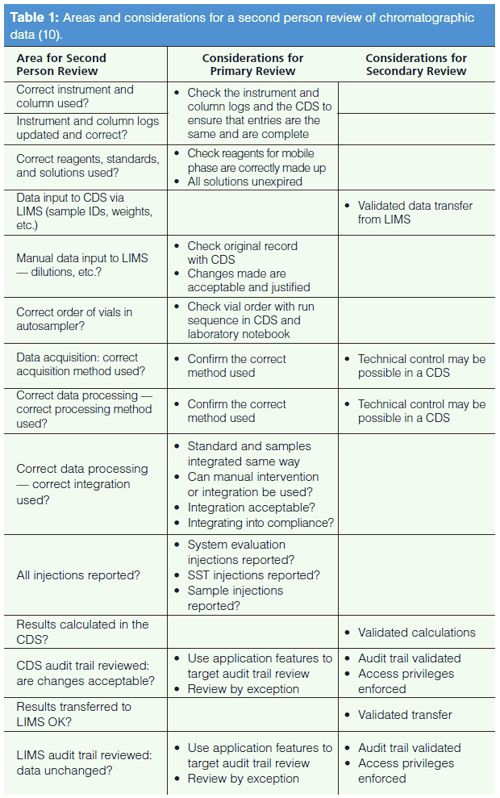
The areas for review are listed in the left-hand column of Table 1 and the areas for consideration for this review are shown in the centre and rightâhand columns. The review is divided into primary and secondary areas. Primary is where the focus should be each time a second person review is carried out and secondary is where technical controls and validation provide support so that poor data management practices or falsification cannot be performed. This does not mean that a reviewer ignores these areas but it allows a risk-based approach to help focus the review on areas that are not enforced by the electronic workflows in the software or where there is manual input to a system. These are areas where poor data management or errors can occur.
Ideally, the applications can help with the review. For example, a CDS application has a function where the audit trail entries can be reviewed, and by pressing a button the name and time stamp of the reviewer can be entered into the audit trail for the sample. Other means of helping the review process are for changes to be highlighted in the audit trail by a colour or other means and the reviewer focuses their attention there.
Summary
This column has looked at the ways a LIMS can be used to help ensure data integrity within a regulated laboratory. The best way is to work electronically and eliminate paper as much as possible. This allows for a more efficient laboratory as well as software applications, enforcing the correct ways to work alongside regulatory compliance to ensure data integrity.
References
- MHRA GMP Data Integrity Definitions and Guidance for Industry 2nd Edition. Medicines and Healthcare Products Regulatory Agency, London, UK (2015).
- Draft Guidance on Good Data and Record Management Practices. World Health Organization, Geneva, Switzerland (2015).
- FDA Guidance for Industry Data Integrity and Compliance with cGMP. Silver Spring, MD, USA (2016).
- J. Donath and R.D. McDowall, LCGC Europe 19(9), 453–464 (2005).
- C.Burgess and R.D. McDowall, LCGC Europe28(11), 621–626 (2015).
- 21 CFR 211 Current Good Manufacturing Practice for Finished Pharmaceutical Products, Food and Drug Administration, (Sliver Spring, MD, USA, 2008).
- Able Laboratories Form 483 Observations (2005). Available from: http://www.fda.gov/downloads/aboutfda/centersoffices/officeofglobalregulatoryoperationsandpolicy/ora/oraelectronicreadingroom/ucm061818.pdf
- EU GMP Annex 11 Computerized Systems, (European Commission, Ed., Brussels, Belgium, 2011).
- 21 CFR 211 Current Good Manufacturing Practice for Finished Pharmaceuticals, (US Government Publishing Office, Washington, DC, USA, 2008).
- R.D. McDowall, Validation of Chromatography Data Systems: Ensuring Data Integrity, Meeting Business and Regulatory Requirements (Second Edition, Royal Society of Chemistry, Cambridge, UK, in press).
“Questions of Quality” editor Bob McDowall is Director at R.D. McDowall Ltd, Bromley, Kent, UK. He is also a member of LCGC Europe’s editorial advisory board. Direct correspondence about this column should be addressed to the editorâin-chief, Alasdair Matheson, at “Questions of Quality”, LCGC Europe, Hinderton Point, Lloyd Drive, Ellesmere Port, CH65 9HQ, UK, or e-mail amatheson@advanstar.com

What Goes in a CDS IT Service Level Agreement?
Published: April 7th 2025 | Updated: April 7th 2025Protecting your network chromatography data system (CDS) data is critical and a service level agreement (SLA) with your IT provider is vital. What should be included? Are SLAs for in-house IT and SaaS (software as a service) similar?






