A History of Gas Chromatography: My Early Experiences
LCGC North America
Industry veteran and 2009 LCGC Lifetime Achievement Award Winner Harold McNair writes about the early days of gas chromatography and his experiences through the years.
I was lucky to be around in the beginning of gas chromatography (GC). I heard about it in 1956, made my first injections in 1957, and am still working with it today. I was recently encouraged by LCGC's Editor-in-Chief David Walsh to write up some of the early history that I experienced, which I present here.

Modern GC was invented in 1952 by Martin and James. Griffin and George (London, UK) probably manufactured the first commercial GC system in 1954, and several companies, including Perkin Elmer, Fisher/Gulf, Barber Coleman, Podbelniak (all U.S.-based) and Pye Unicam (UK), followed shortly in 1955 and 1956 (1).

Harold McNair
I was a summer intern at American Cyanamid in Stamford, Connecticut, in 1956 when our group leader described an amazing new technique (GC) reported by Dr. A.J.P. Martin at a recent Gordon Research Conference. I was fascinated by his report and wanted to learn more. By the next summer (1957), I was an intern at the Amoco Refinery in Whiting, Idaho, packing GC columns, evaluating new liquid phases, and making injections into a homemade GC system with a thermal conductivity detection (TCD). I also assisted Dr. Martin as a "gofor" one day that summer installing his gas density balance. I returned to Purdue, building my own GC system (Figure 1) with a thermal conductivity detector and a glass-thermostated oven for the entire system (compliments of Dr. Steve Dal Nogare, E.I. DuPont, Wilmington, Delaware).
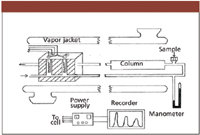
Figure 1: Schematic of a homemade GC system (Purdue, 1957).
In 1959, Dr. Herb Brown, Purdue University (West Lafayette, Indiana) bought a Perkin-Elmer model 154 GC system, the first commercial system at Purdue. Commercial systems were slow to be introduced in the U.S. compared to Europe. I was fortunate enough in 1959 to receive a Fulbright fellowship to study with Prof. A.I.M. Keulemans at the new Technical University in Eindhoven, The Netherlands. Prof. Keulemans had come from Royal Dutch Shell laboratories in Amsterdam, where he had introduced GC early on and had written one of the first comprehensive GC books in 1957. Both Royal Dutch Shell, Amsterdam, and British Petroleum, Sunbury on Thames, were hot centers of GC development in those early years. The petroleum industry was the first to rapidly embrace and develop GC.
I was very lucky to be in Holland in 1959 and 1960. Prof. Keulemans was actively involved with all the major workers in GC in Europe. Many of them came to work in our laboratory in Eindhoven. Dr. Marcel Golay taught both myself and Carl Cramers (later Prof. Dr. C.C.) how to make capillary GC columns using both stainless steel and polymeric tubing. Later, Dennis Desty from British Petroleum installed a glass drawing machine and we began to (not easily) make glass capillary columns. Glass could be made more inert than stainless steel, but it was not easy to manufacture or deactivate. We had several homemade GC systems (shell design) and later one Perkin-Elmer system with TCD. Prof. Keulemans also brought plans from Shell and we constructed our own flame ionization detection (FID) system.
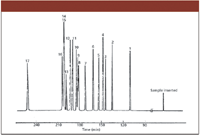
Figure 2: High-resolution capillary GC chromatogram from 1960. This is all 17 octane isomers, a separation that many thought was not possible.
I traveled to England on behalf of Prof. Keulemans several times, reviewing a joint project with Dr. Jim Lovelock on ionization detectors using ultrapure helium and argon supplied by the Physics Lab of Phillips Electronics in Eindhoven and picking up an argon ionization detector, using 90Sr (radioactive!) as the ionization source from Pye Unicam in Cambridge. Cramers and myself presented our first paper on comparing our homemade flame ionization detector and the Pye argon ionization detectors at the Third International GC Symposium, Edinburg, UK, in June 1960. A large number of European contributions in GC were presented at that symposium.
I returned in late 1960 to Esso R&D (currently Exxon) in Linden, New Jersey, Bayway Refinery. They were heavily into GC, using both Perkin-Elmer model 154s and F&M Scientific models 300 and 500. One problem was analyzing "Gasoline of the Day." Because both temperature and flow rate fluctuated during the day and night, we could not reproduce retention times well enough to use the technique for qualitative analysis. I came up with a big rubber band with markers and names of the compounds of interest. All refinery shifts (three per day) would pin down the start of the rubber band on the point of injection on the finished chromatogram (a 3- or 4-ft roll of chart paper). The rubber band was then stretched out until the bright red mark (benzene) matched the largest peak on the paper and was then pinned down. The operators then wrote down the names from the rubber band onto the chromatograph. This proved the concept of relative retention times being reproducible.
I introduced capillary GC to Esso, using both homemade and purchased columns from Perkin-Elmer (stainless steel). Esso was selling wax from its crude oil residue for coating milk cartons. FDA pressured us to rapidly develop a sensitive method to measure parts-per-million levels of polynuclear aromatics (PNAs) believed to be carcinogenic. This was not possible with the current methods using TCD. Having played and studied temperature-programmed GC at Du Pont in 1958 (Dr. Steve Dal Nogare), I developed a large-volume injection technique, with a 100-μL total volume by injecting 25 μL/s four times of a wax sample at low temperature (75 °C), then rapidly programming to elute parts-per-million of PNA. I knew if we started cold at 75 °C, the hexane solvent would be eluted rapidly, but the trace high-boiling PNAs essentially would be frozen on the top of the column. Only when a much higher temperature was reached would they begin to migrate down the column. Thus, I was able to inject 100 μL of a hexane solution, allow the solvent to be eluted, and only then begin temperature programming (Figure 3).

Figure 3: Large-volume-injection ppm analysis of PNAs.
Dr. R. Gohlke had introduced the first GC–mass spectrometry (MS) experiment in 1959 using a packed column (2). But in 1960, no one in our New Jersey discussion group or the recently introduced A.S.T.M Committee E-19 on GC felt that MS could possibly be used with capillary columns because they required microgram and nanogram quantities of sample (the standard technique was to inject 1 μL and split 100:1). Dr. Phil Klass (our Esso resident MS expert) and myself disagreed. We reported what I believe to be the first description of a capillary GC–MS experiment (3). I cannot find the chromatogram and this work was never published in more detail.
My last assignment at Esso was to purchase one of every available commercial gas chromatograph (about 12 models from about six or eight suppliers), evaluate them for Esso refinery and research operations, and make recommendations for which model should be used for which refinery in Esso plants around the world. This was a fun job. The basic conclusions were to use an F&M model 500 with TCD for most high temperature complex work, an F&M model 609 (FID) for trace work, and a Perkin-Elmer model 154 with both TCD and FID for all work outside the U.S. I felt F&M made a better instrument, and certainly for the petroleum industry, their temperature-programming capability and high temperature limits were essential. But outside the U.S., Perkin-Elmer, who also made a good instrument and had a well-established name in laboratory instrumentation, had better sales and service support. Perkin-Elmer had international sales and service offices covering Europe and dealers in many other countries.
I left Esso in December 1960 to set up F&M Scientific's operation in Europe as "Technical Director" — actually I was a salesman, serviceman, secretary, and "boss" in the initial months, but it became a very interesting adventure.
But why did I leave Esso? It was a great job. The answer today seems almost silly. They promoted me to manager of the refinery QC laboratory. This laboratory ran three shifts a day, seven days a week. I was a scientist, and I didn't want to be a manager. A big Esso "boss" from New York City also told me that research and development for Esso was only window-dressing, mostly for stock holders. What R&D did was not that important, only the price of crude oil from the middle east ($4 per barrel in 1961!).
I considered academia, and in fact, I gave a seminar at the Massachusetts Institute of Technology (Cambridge, Massachusetts). A colleague of mine there told me they were looking for an "analytical chemist." They had just lost their only one to Purdue. He also told me they planned to hire two instructors at a low salary, and after a year or two, fire one and promote the other one. I stopped looking for an academic position.
I knew F&M Scientific. I had worked for Dr. Steve Dal Nogare in the summer of 1958. The three founders of F&M all worked in that same group and during my year at Esso, I had spent a lot of time with them in Avondale, Pennsylvania, discussing flame ionization detectors.
After my review of available GC systems, I knew F&M made a good GC system, possibly the best one. Their model 500 was the only high-temperature model, and I thought it was the only temperature-programmed gas chromatography (TPGC) system. I was not aware that Aerograph (Walnut Creek, California) also made a TPGC system. I felt F&M easily could become a market leader if they developed a strong international group, and they were asking me to set up, with their support, their European operations. They wanted an American (whom they could trust), but he also had to speak at least one European language and have some European experience. I fit the bill. Finally, the prospect of returning to Europe with a good American salary was exciting. Fortunately, my wife agreed. I was to spend the next seven years in the instrumentation business before coming to Virginia Tech in August 1968. As you can see, I have many good memories of the early days of GC.
Harold McNair is Professor Emeritus with Virginia Polytechnic Institute, Blacksburg, Virginia.
References
(1) A.T. James and A.J.P. Martin, Biochem. J. 50, 679 (1952).
(2) R.S. Gohlke, Anal. Chem. 31, 534 (1959).
(3) N.Brenner, International GC Symposium, (Academic Press,New York, 1961), p. 537.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











