Highlights of HPLC 2012
LCGC North America
A review of the trends at this year&s symposium, including discussions of column technology, sample preparation, and detector usage.
HPLC 2012 was held June 16–21 in Anaheim, California for the first time in its long history. In this installment, coverage of some of the technology and application advances is reported. We review the overall liquid-phase chromatographic trends observed at the symposium, the opening plenary session, awards presented, column technology highlights, and sample preparation and detector usage.
The 38th International Symposium on High Performance Liquid Phase Separations and Related Techniques, which alternates between Europe and North America with occasional side meetings in Australasia, was held June 16–21, 2012, in Anaheim, California for the first time (not far from Disneyland). More affectionately known as HPLC 2012, the symposium is the premier scientific event for bringing together the myriad techniques related to separations in liquid and supercritical fluid media. Chaired by Professor Frank Svec of the Lawrence Berkeley National Laboratory in Berkeley, California, HPLC 2012 assembled 830 participants from all over the world. This number included vendor representatives (150) from 52 exhibitors for the three-day instrument, software, and consumables exhibition. Based on the lower number of attendees and exhibitors compared to HPLC 2011 in Budapest, the worldwide economic crisis may have played a role in the support for this important conference.
The five-day-plus event had a total of 117 oral presentations in plenary and parallel sessions and 443 posters in sessions with 19 different themes. Attendees had their hands full deciding how to allocate their time with an ample social event schedule including a symposium banquet at Disney California Adventure, nine vendor workshops, six "sunrise" tutorial educational sessions, and seven short courses (held during the previous weekend). The tutorials were particularly well attended and covered current topics such as organic monoliths, selectivity optimization, hydrophilic interaction liquid chromatography (HILIC), higher order protein structure, capillary LC and ion chromatography (IC), and supercritical fluid chromatography (SFC).
Trends in Liquid Phase Technology and Techniques
Obviously, high performance liquid chromatography (HPLC) was the predominant technology in the technical sessions at the symposium, but sample preparation, the use of electrophoretic techniques (mostly in a capillary format), and an increase in method development and method transfer papers were strongly evident. From a perusal of the poster and oral presentation abstracts and notes from my colleagues, I broke down some of the major areas of coverage in this year's symposium. These tables are useful to spot trends in the technology, applications of liquid phase separations, and detection principles that were introduced in this series.

Table I: HPLC 2012 papers presented by technology or technique
Table I provides a rough breakdown of the coverage of liquid-phase technology and techniques in the separation sciences. Compared to HPLC 2011 (1), some slight shifts in technology emphasis were noted. Again this year, new developments in column technology led the pack by far with more than a third of the oral and poster papers dealing with new phases and formats. Surprisingly, nearly 40% of the columns' papers dealt with monoliths. Although not yet considered a commercial success, research interest in this technology is still running high, especially in academia. The polymeric monolith segment is less covered by intellectual property rights than the silica monolith segment. Second-generation silica gel–based monolith commercial products feature better efficiency but slightly higher pressure drops because of the change in the macropore/mesopore domain ratios. Presentations on polymer-based monoliths outnumbered silica-based monoliths by a 2.5:1 margin. This surprisingly high margin may have reflected the bias of the symposium chair, who is a leader in this area of polymeric monolith technology. However, a continuation of new developments in polymeric monoliths devoted to the separation of small molecules has shown improvements in column efficiency; originally, the small molecule domain was for the silica-based monoliths and polymeric monoliths were thought to be useful for macromolecules only.
Three other "hot" areas in column technology this year were
- More emphasis on the new breed of superficially porous packings (SPP, also referred to as shell particles, poroshell, and fused-core packings) that rival the sub-2-µm particles in terms of column efficiency, but with substantially lower pressure drops. The poster and oral papers referring to SPP outnumbered those devoted to sub-2-µm totally porous particles by 60%.
- The widespread attention to HILIC for the separation of polar analytes that are weakly retained by reversed-phase chromatography. This year, just for fun, I tabulated the various LC modes reported on at HPLC 2012 (Table II). Clearly, as expected, reversed-phase chromatography was the number one application mode but HILIC was a fairly strong number two.
- The incorporation of nanoparticles (embedded) into packing media seemed to generate a lot of research interest, especially in polymeric media where the use of gold and carbon nanoparticles imparted higher surface areas (therefore increased capacity) and enhanced selectivity. The nanoparticles themselves can be modified in situ with various functional groups and then used for separations of proteins and other molecules by selective interactions.
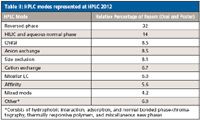
Table II: HPLC modes represented at HPLC 2012
The interest in new research into sub-2-µm porous packings has waned a bit this year, probably because these columns now are firmly established in the ultrahigh-pressure liquid chromatography (UHPLC) world with more than 30 vendors supplying these products. On the other hand, there were considerable discussions about the practicality of whether the next generation of smaller totally porous particles or superficially porous particles will approach the 1-µm dimensions.
Sample preparation technology was well represented in the poster papers and two oral sessions were devoted to some of the current techniques. Most prominent were improved solid-phase-extraction (SPE) and solid-phase microextraction (SPME) technologies with new phase chemistries: monoliths, immobilized enzyme in situ reactors, molecularly imprinted polymers (MIPs), dispersive-SPE phases for QuEChERS (quick, easy, cheap, effective, rugged, and safe), and mixed-mode phases providing new selectivities.
Electro-driven separation techniques (for example, capillary electrophoresis [CE], capillary zone electrophoresis [CZE], micellar electrokinetic chromatography [MEKC], and isoelectric focusing [IEF]) showed a slight dropoff compared to HPLC 2011 (1), but continued to see a strong following in the application papers with great strides being made in interfacing to mass spectrometry (MS). The developing interest in biopharmaceuticals has spurred a renewed interest in CE technologies for the separation of biomolecules. A continued lack of interest in capillary electrochromatography (CEC) was noted with only nine presentations at HPLC 2012.
Areas of Application
Table III is a breakdown of the most popular application areas reported at HPLC 2012. Oral and poster presentations on proteomics, biomarkers, protein, and peptide separations and identification again constituted about one-quarter of all the papers presents. Within these life science domains, areas of major applications were the separation and study of glycosylated proteins (glycans), monoclonal antibodies and antibody fragments, protein aggregates (size-exclusion focus here), and the continuing search for biomarkers with LC–MS-MS leading the way. The glycans are cleaved from the glycoproteins by specific enzymes. Glycan analysis using carbon-based packing materials saw a number of presentations. Pharmaceutical and biopharmaceutical assays in drug discovery, therapeutics, formulations, and active pharmaceutical ingredients (APIs) had wide coverage this year, as usual in the number two position. Other life science applications include the areas of nucleic acids, DNA, and oligonucleotides as well as oligosaccharides and amino acid analysis. Another application area with a strong showing was the use of liquid-phase techniques in foods, beverages, and food safety.
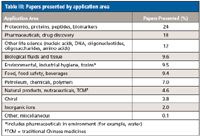
Table III: Papers presented by application area
In this installment, I present some additional scientific highlights from HPLC 2012, including the various awards and honorary sessions that took place. Because it was virtually impossible for one person to adequately cover all oral and poster papers, my coverage will somewhat reflect a personal bias; I was able to get presentation notes from some of my colleagues, who are acknowledged at the end of this column installment.
Awards and Honors at HPLC 2012
Horváth Award: For the seventh year in a row, the Horváth award sessions, named for the late Professor Csaba Horváth, one of the founders of this series and a mentor of young scientists, were featured. This award, supported by HPLC, Inc., a nonprofit group under the guidance of the Permanent Scientific Committee, was established for young scientists in the separation sciences under the age of 35. The award is based on the best oral lecture presented in the Horváth sessions, and the winner is selected by a jury named by the Permanent Scientific Committee. The award consists of a cash prize, invitation to present an oral at HPLC 2013, and a crystal trophy. This year there were 10 nominees, all with strong research credentials. This year's winner was Stefan Bruns of the Philipps-Universität in Marburg, Germany (Figure 1). His coauthor and professor of this award-winning presentation was Ulrich Tallarek. The title of the paper was "Slurry Packing Parameters and their Influence of Capillary Column Morphology." Slurry packing of particles in LC columns has always been considered a form of "black magic," and this research group set out to provide more of a scientific basis for determining the packing microstructure of columns with a focus on the newer superficially porous (core–shell) particles. Using simulations of dispersion to establish a morphology–transport relationship, the workers used confocal laser scanning microscopy to reconstruct segments of capillary columns packed with SPP materials. Using slurry packing, they experimentally investigated particle size distribution, particle surface roughness, column inner diameter, and how these variables affected packing morphology and separation efficiency. The results showed that transcolumn eddy dispersion was of paramount importance and furthermore showed strong evidence of an improved packing structure at the particle-column wall interface which reduced the impact of wall effects.

Figure 1: The 2012 Horváth award winner, Stefan Bruns (left) of the Philipps-Universität in Marburg, Germany, with John Frenz (right) representing the Permanent Scientific Committee.
Poster Sessions and Best Poster Awards: The mainstay of HPLC 2012 was the poster sessions where more detailed applications and methodology studies were reported, often in very specific areas, and face-to-face discussions with the authors were conducted. Fortunately, many of the poster authors were kind enough to provide small reproductions of their poster papers that could be taken for later perusal. Some authors collected business cards and addresses for sending poster reprints by mail or e-mail. Compared to HPLC 2011 (1), the number of posters was greatly reduced, roughly 40% fewer. Reports were that the numbering system was a bit confusing so viewers sometimes had to hunt for the posters of interest. Despite this slight problem, the posters were up for three days, which gave viewers plenty of time to find them. It was noted that about 10–15% of the posters indicated in the abstract booklets were "no shows."

Figure 2: Best Poster Award winners (left to right): Elisabeth Haller, University of Vienna (Vienna, Austria); Benjamin Rogers, Purdue University (W. Lafayette, Indiana); Franta Foret (for Karel Kleparnik), Institute of Analytical Chemistry ASCR (Brno, Czech Republic); Gerard Rozing, Agilent Technologies (Waldbronn, Germany) and co-chairman of the Poster Committee; Siyang Li, The Barnett Institute, Northeastern University (Boston, Massachusetts); Jiri Urban, University of Pardubice (Pardubice, Czech Republic); Axel Vaast, Vrije Universitiet Brussel (Brussel, Belgium); Michelle Camenzuli, University of Western Sydney (Parramatta, Australia); Peter Schoenmakers, University of Amsterdam (Amsterdam, The Netherlands) and co-chairman of the Poster Committee; Jana Krenkova, Institute of Analytical Chemistry ASCR (Brno, Czech Republic); Arianne Soliven, University of Minnesota (Minneapolis, Minnesota); and Siarhei Khirevich, Philipps-Universitat (Marburg, Germany).
The poster committee cochairs at HPLC 2012 were Professor Peter Schoenmakers, a member of the Permanent Scientific Committee from the University of Amsterdam in The Netherlands, and Dr. Gerard Rozing of Agilent Technologies in Waldbronn, Germany. The 59 members of the poster committee devoted a great deal of time and worked very hard to narrow down the huge collection of posters by the end of the third day to 30 finalists. Eight new reviewers were chosen to help select the 10 winners by Thursday afternoon of the symposium. The selection criteria were based on three factors: inspiration (creativity, newness, uniqueness, and originality), transpiration (experimental execution and completeness of work), and presentation (overall readability, visual impression, and the author's explanation). The winning posters were viewed by all of the final eight committee jurors.
The Best Poster Awards, sponsored by Agilent Technologies, were announced at the closing session. The Poster Award winners for HPLC 2012 are shown in Figure 2 and their poster titles are listed in Table IV. All of the winners received a $500 cash prize. Because of the large numbers of awards granted, a detailed technical coverage of each of the award-winning posters cannot be provided. It suffices to say that all the winners should be proud of their accomplishments because they represent the top 2% of all posters presented at HPLC 2012.

Table IV: Best poster winners at HPLC 2012
Opening Session and Plenary Lectures
Opening ceremony plenary lectures, presented on the first evening of the symposium, are supposed to be cutting-edge, thought-provoking presentations that inspire attendees to think outside the box. After the "Welcoming Remarks" by Dr. Frank Svec, two notables in the field of biopharmaceuticals presented plenary lectures. The first speaker was Steven Kozlowski of the United States Food and Drug Administration's (FDA) Center for Drug Evaluation and Research (CDER) in Silver Spring, Maryland. His talk was entitled "Regulatory Challenges for Biologics: Biosimilars, Characterization & QbD" and covered the regulatory aspects of biosimilars. CDER's best-known job is to evaluate new drugs before they can be sold, including biosimilars. The second talk was by Professor Barry Karger of the Barnett Institute at Northeastern University in Boston, Massachusetts. Karger spoke about recent advances in LC–MS for translational regulatory science.
Both plenary talks highlighted the need for effective and predictable regulatory characterization pathways. With more than 36 pre-investigational new drug (IND) requests for proposed biosimilars alone, both speakers discussed the many challenges associated with careful classification of therapeutics and the need for developing robust methods and protocols for compliance. Karger's talk was more integrated with LC–MS and regulatory requirements, and Kozlowski focused on approaches considered by the FDA in ensuring pharmaceutical quality such as quality by design (QbD), current good manufacturing practices (cGMPs) for the 21st century, and the process analytical technology initiative. He further defined a desirable biosimilar as having no clinically significant difference between it and the biological drug although a slight difference in rate of occurrence in adverse effects will not make it a dissimilar drug. Subtopics in their talks such as "How close is close enough?" and "Structural and functional characterization pyramids" are a sampling of how challenging this analytical science is compared to traditional small-molecule discovery. One example indicated more than 30,000 potential structure modifications on an antibody alone. It is clear that biotherapeutics characterization will require myriad analytical techniques depending on the discovery and manufacturing path and that the interest and effort towards biopharma characterizations will continue to grow and expand.
The remainder of bio-oriented oral presentations and posters at HPLC 2012 supported the opening plenary talks by displaying very comprehensive characterization methods among many modes, but primarily size-exclusion chromatography (SEC), reversed-phase chromatography, and ion-exchange chromatography (IEC). The extensive coverage on MS detection and characterization of biomolecules was evident in numerous presentations.
New Column Technology Highlights
As seen in Table I, the development and study of columns and stationary phases still dominates new technologies. Both oral sessions and poster sessions primarily devoted to this area included sessions on packed-column technologies (three sessions), monoliths (three sessions), particles and highly ordered structures, ion chromatography, modeling and theory, HILIC, imprinted sorbents, ionic liquids, and chiral separations. If one combines all papers pertaining to column technology, more than a third of the presentations at HPLC 2012 covered this topic. Despite all the advances made in column technology to date, investigations on further developments in academia as well as the commercial side are still taking place.
Superficially Porous Particles
From observations made at HPLC 2012, a continuing "hot topic" in HPLC column technology is the discussion of the alternative approaches for developing faster separations and generating more column efficiency at lower pressure drops and which approach will be favored in the long run. Two years ago, the focus was on comparing sub-2-µm totally porous columns to porous particles in the 2–3 µm range and larger. One year ago, the discussions were focused on the superiority of superficially porous columns over sub-2-µm particle columns. This year's focus was mostly on the superficially porous particles (SPP) themselves since they provide the efficiency of sub-2–µm particles but at around half the operating pressure. I counted a total of 47 papers devoted to SPP columns. There is still some confusion about the nomenclature, and during HPLC 2012, these particles were referred to as SPP, fused-core, shell, porous shell, and poroshell particles. It is now generally accepted that SPPs bring the greatest advantage to users compared to porous particles. Monolithic columns, which if improved further, could become a major competitor to the SPP columns. The only question now is: Should both larger and smaller (perhaps down to 1-µm dp) SPPs in various pore sizes and different selectivities be made available? For example, it was demonstrated in the paper of Way and Santasania (2) that 5-µm SPPs provided 60% more efficiency than totally porous 5-µm particles. Table V gives an update on the current status of commercial SPP products as of HPLC 2012 for large and small molecules.

Table V: Commercial superficially porous particles - status as of HPLC 2012
Although users who have already purchased UHPLC equipment are less concerned about the lower pressure drops of these columns, for difficult separations long columns and lots of theoretical plates are always in demand. Therefore, pressure availability is always a concern. Currently, the SPP columns on the market do not have the upper pressure limits of some of the UHPLC instruments but that should change in the near future.
Several interesting papers on the SPP approach were presented during the packed-column technologies sessions. A Monday morning presentation by Georges Guiochon and Fabrice Gritti of the University of Tennessee in Knoxville, Tennessee, discussed the challenges encountered in performing ever faster, more efficient, yet practical separations. Professor Guiochon, who has probably studied the performance characteristics of SPP more than most researchers, focused on describing the ultimate limits of performance that could be expected for smaller particles with a particular focus on SPP. Using small molecules and small proteins (up to 20 kDa) and macromolecules (200 kDa and higher) as model compounds, he described the linear velocities and plate heights that could be expected if particle diameters were to go down as low as 1 µm with "crust" layers with pore sizes in the 150 Å and 300 Å ranges. The latter size provides a sufficient pore size for macromolecule separations. He concluded that the necessary linear velocities for small molecules would generate pressures at 4000 bar or higher and that it would not be practical at the typical linear velocity of 2 cm/s, but for intermediate (0.1 cm/s) or low velocities (0.01 cm/s), the latter preferable for macromolecules, the smaller particles would be practical and possible. In addition, the heat friction generated at higher flow rates and pressures may dominate the separation mechanisms, giving rise to reproducibility problems. The problems of efficiently packing particles in the 1-µm range may also cause headaches. Guiochon called on industry to address the instrumental extracolumn dispersion that may limit the performance of the next-generation columns. Short, simple channels to connect the injector–column–detector combination is paramount to good efficiency. He further called on industry to increase data acquisition rates in advance of the coming smaller particle sizes.
A follow-up talk by Monica Dittmann and coworker Konstantin Choikhet from Agilent Technologies in Waldbronn, Germany, further addressed the problem of extracolumn contributions to small and microbore HPLC columns in both isocratic and gradient separations. To experimentally determine the dispersion in individual system components such as connection capillaries, detector cells, or injection devices, the authors designed an ultralow-dispersion system with a system variance of 0.2 µL. This system also allowed the determination of column-generated peak variances, keeping external contributions at an ultralow level. As might be expected, the extracolumn band broadening was more significant for low k values and at k values of 10 and higher no significant differences in peak efficiency among various column diameters were noted. The dispersion contributions of various connection capillaries, detection cells, and autosamplers were determined as a function of flow for different solvent compositions. Showing that some of the measurements change with flow rate, she concluded that systems must be carefully plumbed with the smallest practical internal diameter tubing (with smaller internal diameters resulting in considerable back pressure despite improved dispersion) and flow cells, and also stating that for isocratic, 1-mm i.d. separations the situation was inevitably the worst case, but 2.1-mm i.d. gradient work was far less challenging.
The possibility of even smaller SPP particles was explored by Tivadar Farkas of Phenomenex in Torrance, California. Although the feasibility of constructing SPP particles with diameters of 1–1.2 µm has been shown, formidable challenges remain. Firstly, the column pressure would be approximately 2.3 times higher than with the smallest currently available 1.7-µm dp SPP columns, which operate with the same pressure drop as 1.7-µm totally porous particles (TPP). Reproducibly packing efficient columns may be a challenge. Frictional heating would increase by roughly the same 2.3 times, although the heat capacity of SPP have been shown to increase compared to that of TPP of the same diameter. Most likely, elevated temperatures would be required to decrease mobile-phase viscosity and the operating pressure. Extracolumn effects of current instruments would need a considerable reduction to realize the true efficiency of the powerful columns. Nevertheless, Farkas showed some van Deemter curves of columns with convincing data that reduced plate heights in the 1–2 range might be achieved, giving something like 500,000 plates/m but with an astronomical pressure drop.
A separate paper by Fabrice Gritti discussed the impact of eddy dispersion on column performance. For modern packed-bed columns like SPP and sub-2-µm columns, eddy dispersion accounts for as much as 75% of the total plate height with mass transfer and axial diffusion contributing much less than originally thought. Eddy dispersion accounts for the contribution to band broadening originating from all sources of flow unevenness in the mobile phase percolating through a column. Gritti's practical and theoretical studies showed that eddy dispersion is mostly controlled by end and wall effects. Using SPP spherical packings with narrow particle-size distributions that are well packed in smooth-walled columns might help to lower eddy dispersion effects and, when used with the future wave of low-dispersion instruments, could result in better performance for everyone.
An interesting paper by David McCalley from the University of West of England in Bristol, UK, and colleagues compared overloading behavior of strongly acidic and basic probes for both SPPs and sub-2-µm TPPs in reversed-phase LC. Despite the shell particles containing a smaller volume of porous packing, similar overloading behavior was found for both materials. The authors studied low to high solute concentrations at different pH values. All the columns gave good peak shapes with small concentrations, but ionized acids and bases gave rapid overloading of all columns that was dependent on pH. Some interesting and perhaps unexplained observations were noted such as 4.6-mm i.d. columns giving lower loading than 2.1-mm i.d. columns and an increase then a decrease in efficiency with sample loading for basic compounds at mid-pH. The latter explanation may be related to the presence of ionized silanols at mid-pH values.
The very first modern wide-pore SPP was the Poroshell 300 stationary phase (Agilent Technologies) introduced by Jack Kirkland in the early 2000s. These SPPs proved useful for the fast separation of proteins and larger biomolecules. The particles' 300-Å pore diameter was sufficiently wide to accommodate most proteins commonly encountered. Now, wide-pore SPPs are being reinvestigated by several research groups. At HPLC 2012, Kirkland and coworkers reported on their work on SPPs with larger pore sizes than the current 90 Å used for small-molecule separations. They investigated a number of parameters that may affect the separation of larger molecules, such as particle size, pore size, shell thickness, and ligand length. Three shell particles were examined: 2.7-µm core with a shell thickness of 0.35 µm; 2.7-µm core with a shell thickness of 0.2-µm; and 3.4-µm core with a shell thickness of 0.2 µm. Using ribonuclease (13.7 kDa) as a test solute, van Deemter curves showed that the larger particle provides lower h values than the smaller particles did. Presumably, the larger particle size packed more efficiently in the column. Different pore sizes were examined: 90, 160, and 400 Å. The smallest pore size showed restricted access for solutes with molecular sizes of 5000 Da and higher. The intermediate pore size could accommodate molecules as large as 15,000 Da before peak shape was affected but the largest pore size showed no restriction for most proteins. The 2.7-µm, 400-Å particle gave better efficiencies than a totally porous 300-Å particle when tested under the same conditions. They also investigated alkyl chain lengths of bonded phases and found that C4 and C8 gave superior performance compared to C18. A rabbit skeletal myosin sample gave better resolution of light chain (17 kDa) and heavy chain (220 kDa) monoclonal antibodies on the 400-Å, 2.7-µm particles than on the 160-Å, 2.7-µm particles.
Monoliths
Monolith columns have been desirable for a long time because they exhibit high permeability and low pressure drop (because of the increased bed porosity), show reasonable separation efficiencies, have the absence of frits to confine the packing material, are easy to fabricate, and can be made fairly reproducibly. Although this technology has been around for several years, as a routine tool it has yet to see widespread acceptance on the commercial side; however, improvements continue to be made for both polymer- and silica-based monoliths. HPLC 2012 had three oral sessions (11 talks), one tutorial, and 31 posters devoted to the monolith technology. As mentioned earlier, reports on polymeric monoliths outnumbered silica monoliths by a 2.5:1 margin.
In his plenary lecture on the first full day of the symposium, Nobuo Tanaka of GL Sciences and the Kyoto Institute of Technology in Japan gave an excellent overview of the status of monolithic silica columns for HPLC. Monolithic structures allow independent control of through-pore size and skeleton size providing the possibility for higher performance than that of particulate columns. He provided a practical comparison of TPP and SPP columns with first- and second-generation silica monoliths as depicted in Table VI.
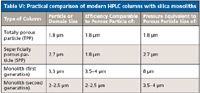
Table VI: Practical comparison of modern HPLC columns with silica monoliths
For small-diameter columns of 2 mm i.d., the performance of monolithic silica columns at 20 MPa or lower pressures can be compared favorably with that of a column packed with sub-3-µm core–shell particles or sub-2-µm TPPs operated at a much higher pressure. Such monolithic columns allow for the use of conventional 400-bar HPLC instrumentation.
Tanaka also discussed the fabrication of monolithic silica capillary columns that allow long columns (for example, 0.1 mm i.d., up to 500 cm in length) for high-efficiency separations utilizing the high permeability of the monolith. One advantage compared to larger diameter columns is that the monolithic structures can be prepared directly in the fused-silica capillary; thus, they do not require cladding after the preparation. Connected capillary columns have already produced 1,000,000 theoretical plates at pressures below 50 MPa.
Research monoliths are one thing, but producing them on a practical scale is another. Commercial silica monoliths are improving but still have not reached the performance of either sub-2-µm TPPs or even 2.7-µm SPPs. However, efforts to improve these products will continue and efficiency will improve, but, because of the tradeoffs mentioned above, pressures will increase.
As Milton Lee of Brigham Young University in Provo, Utah, pointed out in his "sunrise" tutorial on organic monoliths, these stationary phases potentially offer a wider range of chromatographic selectivity, lower pressure drop, and more control over nonspecific sorption in LC compared to columns packed with small spherical particles. However, they currently suffer generally from less homogeneous and reproducible morphology, and lower chromatographic efficiency. Monolith homogeneity depends on the selection of materials and conditions for synthesis, and how well the conditions are controlled.
Compared to silica monoliths, polymeric monoliths are not as rigid; therefore, they are not as stable under pressure. However, they are more varied in their morphology and chemistry and wall effects are easier to control. By synthesizing them, it is harder to generate bimodal pore structure. They do provide symmetrical peaks since there are no silanols around to interact with basic compounds; there is minimal nonspecific interaction. There are many chemistries available and, thus, they provide good selectivity options.
As was seen by the many papers at HPLC 2012, considerable progress is currently being made to develop reproducible, robust, selective, and efficient polymer monoliths for a wide range of applications, including reversed-phase, ion-exchange, hydrophobic interaction, hydrophilic interaction, and size-exclusion chromatography. In the past, polymeric monoliths were confined to separating larger biomolecules but in recent years, researchers have been optimizing their structures to handle small molecules thereby challenging the silica monoliths, TPP, and SPP packings. Novel ways of building selectivity into polymeric monoliths were reported at the meeting. Papers on direct polymerization of functional monomers, chemical modification of preformed monoliths, photolithography of monolithic surfaces, and incorporation of nanostructures within the chromatographic bed were given. The technique of hypercrosslinking allows independent control of pore size distribution of both large, flow-through pores and the small pores in which most of the chemical interactions take place thereby giving users the ability to control the surface area.
An area that several groups are exploring is the study of new material compositions such as titania monoliths, monolithic macroporous calcium phosphates (hydroxyapatite), and copolymeric monoliths such as poly(styrene–divinylbenzene) (PS-DVB) with polymethacrylates and polyacrylamides. An exciting area is the development of hybrid (organic–inorganic) monoliths that may retain the good properties of both approaches. In his paper, Gert Desmet of Vrije Universiteit Brussel in Elsene, Belgium, did some theoretical simulation studies looking at the potential advantage of combining the porous shell approach with silica monoliths. His conclusion was that having a solid core within the monolith brings the same advantage as it does within an SPP. Time will tell if researchers in the monolith columns area can accomplish the task of combining the two concepts on a practical basis.
HILIC
As indicated earlier, HILIC was again a widely discussed technique at HPLC 2012. Not only was there a "sunrise" tutorial lecture by the "inventor" but an oral session was devoted to it and numerous oral and poster papers illustrated the use of the technique, speculated about the mechanism, and developed new phases that exploit the new found advantages of the technique. As a refresher, HILIC is a separation technique for highly polar analytes that gets around some of the problems associated with reversed-phase chromatography such as low retention or phase collapse (dewetting). For polar compounds that are eluted very early or are unretained on a typical reversed-phase column, HILIC is ideal. HILIC uses a polar stationary phase such as bare silica gel, polar bonded phase (for example, diol, amines, and amides), and certain mixed mode or zwitterionic phases. Operating conditions usually require a high percentage of a nonpolar mobile phase, similar to the requirements for normal-phase chromatography. However, unlike normal-phase, which uses nonpolar solvents like hexane and methylene chloride and tries to exclude water from the mobile phase, HILIC requires some water in the mobile phase to maintain a stagnant enriched water layer on the packing surface into which analytes may selectively partition. In addition, water-miscible organic solvents are used. Under HILIC conditions, polar analytes are well retained and are eluted in order of increasing hydrophilicity. Oftentimes, but not always, elution order is the opposite of reversed-phase chromatography. Gradients are utilized where increasing amounts of aqueous in the mobile phase elute the more polar analytes. HILIC is especially favored by mass spectrometrists since ionization efficiency is often enhanced in high percentages of organic solvents and the presence of lower concentrations of volatile buffer salt compared to reversed-phase chromatography.
Andrew Alpert of PolyLC is considered the "father" of HILIC even though he himself admits that people were performing the technique years before he coined the term, separating sugars using polar phases like amines and acetonitrile–water mixtures as mobile phases. Nevertheless, the technique lay dormant for over a decade until phases like ZIC-HILIC (SeQuant/Merck) and Atlantis HILIC (Waters) came onto the market in 2004. Even Alpert admits that there is considerable speculation about the exact mechanism, or perhaps one should say the primary mechanism, of the HILIC separation. The first speculation is that the mechanism is partition. The water layer on the surface of the packing material may be from 4–15% depending on the organic concentration in the mobile phases. Bare silica and diols have the thinnest layers. It is possible that there are up to three layers of water varying in their partitioning capability. When comparing silica to amide phases, the coated amide surface offers superior peak shape. Some columns that exist in a zwitterionic form are similar to strong cation exchange when operated with less than 20 mM salt. Another speculation is that the dominant mechanism is hydrogen bonding, but this is difficult to prove. Electrostatic effects are minor mixed-mode effects in which attraction and repulsion occur relative to the salt molarity. This is normally effective in the 5–40 mM range and, despite the general recommendations of many HILIC column providers, separations are much more robust when the salt molarity is 10 mM and higher, despite many examples from vendors using 5 mM salt. This may, in part, explain why people have difficulty getting good reproducibility from column to column and instrument to instrument.
One interesting example of a zwitterionic material is polyhydroxy ethyl A. Alpert showed an example separation of lysine, tyrosine, and asparagine over the pH range of 3–7. At pH 3, tyrosine was strongly retained and lysine was poorly retained. At pH 7, the opposite was true. Asparagine was unaffected by the pH of the mobile phases, which contained no organic. Returning to the discussion of electrostatic factors, the author explained that ionic propulsion can include a behavior much like SEC where the analyte elution occurs before the void peak. Ion pairing is one way of controlling the orientation of a molecule as it presents itself to the separation surface. One simple example of this is how one may use trifluoroacetic acid to mask the effect of ionic amine functional groups. In the case of amphoteric compounds, it is possible to change the molecule orientation by choosing whether to ion pair the amine group or the acid group.
Wolfgang Lindner of the University of Vienna in Vienna, Austria, discussed selection of the HILIC phase and conditions for operation. Currently, there are around 40 HILIC phases on the market. They range from bare silica, diol, anion and cation exchange (alone or in zwitterionic phases), amide, and urea, to mixed mode (for example, reversed phase–weak anion exchange and reversed phase–weak cation exchange). The salt or molarity is an important aspect of partitioning, in which the organic phase and trapped layers of aqueous (protic) solvent can truly partition. Lindner pointed out a number of shortcomings with HILIC, including: robustness, gradient reproducibility, and analyte solvation which is particularly important. Interestingly, he did not mention longer equilibration times after gradient elution that have been observed by others. The author gave a review of contributing behavior of the mobile phase, the stationary phase, and solute properties in describing the complexities of this mode of separation. In his work, he determined that the order of solvent strength went from acetone (the weakest), to acetonitrile, isopropanol, ethanol, methanol, and finally water (the strongest). One of the possible substitutions for water is ethanediol. An example on a Luna HILIC column (Phenomenex) was shown at this point using ethanediol in the mobile phase.
Lindner showed a very nice orthogonality graph for HILIC vs. reversed-phase modes that was based on a large number of analytes of varying properties. Of all analyte classes shown in the orthogonality discussion, the most dramatic examples in that comparison were peptides where the primary variable was high and low pH on reversed-phase columns.
New Column Technologies
Each year at the HPLC meeting new column designs are presented, and this year was no exception. In a follow-up paper to their HPLC 2010 report on capillary columns packed with silica colloidal crystals entitled "Submicrometer Particles Can Give >1 Million Plates in Protein RPLC for L=2 cm," Mary Wirth and colleagues from Purdue University in West Lafayette, Indiana demonstrated a quantum leap in column efficiency for a test protein (bovine serum albumin [BSA]) compared to incremental gains in plates normally reported. Her colloidal crystal particles were of submicrometer dimensions (470 nm). The observed peak widths for BSA for a pressure-driven 20 mm × 75 µm column was 15-fold narrower than the theoretical limit for Hagen-Poiseuille flow. The zone variance, normalized for separation length is 15 nm, which is 500-fold smaller than previous reports for pressure-driven protein chromatography. This remarkable achievement was attributed to "slip flow," which is depicted in Figure 3. Slip flow in nanofluidics arises from weak intermolecular interactions between a fluid and the surrounding wall, giving significantly enhanced volume flow rates of water through hydrophobic nanoscale channels such as carbon nanotubes, nanopipes, and nanoscale carbon sheets. Slip flow requires that the channel radius approaches the slip length. An exciting outcome of slip flow is the narrowed velocity distribution that is depicted in Figure 3; compared to Hagen-Poiseuille flow, slip flow velocity distribution is narrower across the silica colloidal crystal channels, estimated to be around 30–40 nm. A nice example using one of their columns was the separation of a monoclonal antibody from its aggregate in only 40 s, representing a 10-fold increase in speed over conventional separations of proteins. Thus, slip flow has profound implications for protein chromatography.
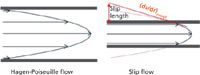
Figure 3: Contrast between slip flow and Hagen-Poiseuille (no slip) flow. Slip flow has only a segment of the parabolic flow profile inside the channel, reducing the velocity distribution.
Some new approaches to bonded-phase technology were reported at HPLC 2012. Boguslaw Buszewski and colleague from the Nicolaus Copernicus University in Torun, Poland, discussed a new phase that mimicked the phospholipids bilayer of cell membranes (pseudomembranes) that was called an immobilized artificial membrane (IAM) phase. It consists of monolayers of phospholipid analogs covalently bonded to the silica surface. The phase more closely mimics the interactions of analytes with biological membranes than a classical C18 phase. The IAMs are useful in pharmaceutical studies because they can be used in modeling drugs and drug candidates' permeation through biological membranes. Buszewski's phases were either cholesterol- or phosphoaminoalkyl-bonded (C10 or C18 alkyl chain length) materials and, aside from mimicking cell membranes, could also be used for other separations in the reversed-phase chromatography or HILIC modes. He noted for the latter bonded phase types, as the ligand density increased, there was a secondary effect of shifting ionic behavior from anionic to cationic.
Hideko Kanazawa and coworkers from Keio University and Tokyo Womens' Medical University in Tokyo, Japan updated their work on temperature-responsive chromatographic phases, this time for the separation of biomolecules. These interesting phases are based on a thermoresponsive poly(N-isopropyl acrylamide) and its copolymer-modified surface. The surface properties and retention of analytes of these polymers are controlled by external temperature changes rather than by mobile phase changes. A purely aqueous phase can be used without any organic solvent present, which is important for the solutes to maintain their biological activity. The driving force for the separations is hydrophobic interaction and electrostatic interactions that can be modulated simultaneously by varying the temperature of the mobile phase. Other interesting stationary phase studies were conducted using MIPs, plastic antibodies, and ionic liquids.
There were also presentations given on the growing interest in ionic liquids (ILs) in chromatographic systems. An excellent and captivating discussion of the background and properties of ILs relative to ecology (are they "green" or not?), flammability, toxicity, and volatility, was given by Maria-Lisa Riekkola and coworkers from the University of Helsinki in Finland. Her talk discussed the valuable properties of these materials and stated that many of their practical and unique aspects were retained when adsorbed or bonded to a usable chromatographic substrate. She described the various ligands that could be used to prepare the ILs with a focus on specificity and capacity. The ILs were used in both SPE and LC formats. In their work, the group prepared several specific ligands bonded to silica, including imidazole and 1-methylimidazole and compared their performance to strong anion exchange, strong cation exchange, and MAX (a unique mixed-mode material, presumably hydrophobic and anion exchange). The bonded ionic liquid phases were found to be pH-stable in the 2–10 range for 24 h. Riekkola's applications of the ionic liquid phases focuses on collection of atmospheric aerosol samples. She noted that the traditional thought around the nucleating agent for airborne polluting aerosols was sulfate and others with ammonia. Now, though, the general feeling is that organic amines are more likely than ammonia. Showing recovery data for the prepared IL materials and strong anion and strong cation exchange SPE phases, she concluded that an IL zwitterionic phase worked the best for acids and amines in collected aerosol samples. They also packed some capillary columns with the materials and ran them as LC columns, showing good selectivity, if poor tailing peak shape, for test analytes. An imidazole IL material behaved similar to the MAX mixed mode material also used for comparison in an LC format.
Multidimensional and Comprehensive Liquid-Phase Separations
There were two oral sessions and one poster session (11 posters) devoted to the topic of multidimensional and comprehensive LC. Once again, multidimensional separations, especially 2D, received the most attention. As samples become more complex, even with the best and most efficient columns available, the peak capacity of a single column is no longer sufficient to resolve all the peaks to baseline. Peak capacities of 1000 may be achieved with a single column in a very long analysis time. To handle samples with thousands and tens of thousands of components, multiple columns with different separation mechanisms are needed. The total peak capacity of a multidimensional system is equivalent to the peak capacity of the initial column times the peak capacity of the second column, if the two chromatographic modes are orthogonal. Peak capacity is the chief metric of resolving power in 2D LC and Peter Carr of the University of Minnesota in Duluth, Minnesota, discussed the limits of peak capacity in his presentation "Comprehensive In-Line 2D LC." He feels that we now have a good understanding on how to optimize 2D peak capacity and minimize its limitations. Carr used the little understood Knox-Saleem limit (how many plates can be generated in given system pressure when particle size, column length, and flow rate are simultaneously optimized) to show how it can be adapted to the theory of gradient elution in 1D and 2D LC and to define the ultimate theoretical limits of both methods.
Dwight Stoll (one of Professor Carr's students) and his coworkers from Gustavus Adolphus College in St. Peter, Minnesota, and the University of Minnesota went on to describe a technique called selective comprehensive 2D LC (sLC×LC), which is designed to enhance the resolving power of selected regions of a conventional separation. Using equipment similar to comprehensive 2D LC×LC, Stoll selectively samples important areas of the first dimension and directs them via a sampling loop to the second dimension where a rapid separation takes place. The experiment is repeated whenever an additional area of interest is eluted from the primary column. Thus, they view sLC×LC as a bridge between the multidimensional methods of heart cutting, which is concerned with the analysis of small numbers of compounds (approximately 1–3) per analysis, and fully comprehensive (LC×LC) separations that are typically focused on the separation and analysis of hundreds of compounds present in complex mixtures. Examples of this technique were presented comparing his approach vs. the use of the same two separate columns. The sLC×LC approach significantly reduces method development time, reduces overall analysis time, and allows selective elimination of time-consuming sample preparation steps.
Orthogonality using all the available space in a 2D plot is what method developers desire from their multidimensional chromatographic systems. Martin Gilar from Waters in Milford, Massachusetts, with colleagues from SUNY in Binghamton, New York, and Dow Chemical Company in Spring House, Pennsylvania, used mathematical methods such as correlation coefficients of various types, mutual information obtained from information theory, the dimensionality obtained from the box counting algorithm, and surface fractional coverage obtained from various hulls such as rectangular and the minimum convex hull. Except for correlation coefficients, the other methods provided a suitable measure of orthogonality. The presented results were discussed in terms of method applicability, simplicity, and utility for real chromatographic data. Two of the methods, box counting and fractional coverage, were found to be mathematically related.
Some practical demonstrations of multidimensional separations were noted. Luigi Mondello and colleagues from the University of Messina in Messina, Italy, and other Italian institutions have been advocates of investigating real-world samples. In this work, they employed SPP particles in the second dimension, which achieved the rapid, low-pressure separations needed to successfully perform LC×LC. Because of the complexity of samples that they analyze, they mainly use MS for detection, adding even a third dimension.
Pavel Jandera and his colleagues from the University of Pardubice in Pardubice, Czech Republic, provided further reports on their use of various combinations of gradients in the first and second dimension. As in 1D chromatography, gradient elution provides significant improvements in peak capacity in comprehensive 2D LC×LC. The best results are achieved when using simultaneous gradients both in 1D (for a constant sampling rate per peak width) and in 2D (for maximum band compression). However, 2D gradients are limited to a short time period. Thus, fast columns are used in 2D (for example, sub-2-µm, monolith, and porous shell columns) as well as fast gradients. The gradients have to be well optimized in both dimensions. The operation conditions are selected to avoid solute carry-over between the subsequent fractions analyzed in 2D and to suppress band broadening or distortion because of limited compatibility of the mobile phases used in the two dimensions. Their favorite modes of coupling in the first and second dimensions are the reversed phase–reversed phase and HILIC–reversed phase modes. In a subsequent paper, Petr Cesla of the same laboratory discussed a new approach for optimization of highly efficient separations using micellar electrokinetic chromatography (MEKC) in the second dimension and columns with octyl- and octadecyl-silica fused-core particles in the first dimension. Some optimization had to be performed by changing the composition of the micellar pseudostationary phase in background electrolyte as well as the gradient change of instrumental parameters (temperature, applied voltage) to compensate the influence of increasing concentration of acetonitrile (from the first dimension) on MEKC separation and to increase the speed of analysis by manipulating the migration window in MEKC. A model describing the migration of analytes under gradient conditions was presented.

Table VII: 2D comprehensive LCÃLC techniques reported at HPLC 2012*
Since there were a large number of papers dealing with multidimensional LC separations, I tallied the types of phases that were used in the first and second dimensions and came up with Table VII, which shows the modes being coupled. In most cases, the mobile phases are readily compatible while in other cases special considerations must be given to coupling incompatible liquids.
Sample Preparation
As covered in Table I, studies and uses of sample preparation technologies were widely prevalent in both lecture and especially poster sessions at HPLC 2012. Two oral sessions and one poster session were devoted to this important step in the analytical cycle. With all the strides made in fast LC, sample preparation has been and continues to be the rate-determining step in throughput. There were many sample preparation procedures reported at HPLC 2012. Here is a short listing of the more important ones:
- SPE — by far the most widely reported technique
- MIPs — very selective, compound-specific extractions
- Immobilized enzymes — for in-line digestion of proteins
- On-line column switching — a way to automate sample prep via valving
- Liquid–liquid extraction (LLE) — a time-proven technique
- Supported-liquid extraction — can replace LLE with many advantages (3)
- Supercritical-fluid extraction (SFE) — great for nonpolar analytes in solid matrices
- Pressurized-fluid extraction (PFE) — for more rigorous extractions of analytes from solid matrices
- Matrix-solid phase dispersion (MSPD) — for extractions using solid-phase sorbents in direct contact with sample
- Electromembrane extraction (EME) — separation of ionic and ionizable substances across a supported liquid membrane
- QuEChERS — mainly for pesticides in fruits and vegetables but expanding beyond
- Protein precipitation — most widely used technique for drugs and metabolites in biological fluids
- Turbulent flow chromatography —fast sample prep of biofluids
- SPME — widely used for semivolatiles and for gas chromatography
- Dried blood spotting — a new way to sample and transport blood samples.
A couple of the more interesting oral presentations will be highlighted here. Janusz Pawliszyn, the father of SPME, of the University of Waterloo, in Waterloo, Ontario, Canada, has been working on new formats that can increase the overall sensitivity of the technique. The SPME materials can exist in various forms, including fibers, tubes, and various flat shapes. His latest innovation has been the development of thin films of adsorptive phase that provide a higher surface area than fibers (and thus high sensitivity) and with the appropriate agitation can provide faster extraction times. This new format has been adapted to 96-well plates and thus allows a higher degree of automation than previously allowed. Even though extraction times are in the 90–180 min range, the fact that 96 samples are extracted at once gives a reasonably fast sample preparation method on a sample-per-minute basis. A newer extraction phase, polyacrylonitrile (PAN), can be blended with C18 or DVB and fashioned into SPME "blades." These new devices were used for the sample preparation of drugs in fish muscle, phenolics in grapes and wine, and pharmaceuticals in wastewater with low limits of detection and typical recoveries in the 73–86% range (for the drugs).
With recent interest turning to smaller samples of whole blood for clinical analysis, Karl-Siegfried Boos of the Medical Center of the University of Munich, in Munich, Germany, reported on a fully automated platform for in-line pretreatment and on-line cleanup of whole blood before LC–MS-MS analysis. After reviewing the current methods for dealing with blood samples, he focused on the growing technique of dried blood spotting (DBS) and some of its disadvantages (for example, laborious spot punching, the need for exact blood volume, the addition of internal standard in later stages of the process, and the time-consuming drying process). He covered approaches now being used for the automation of the technique, mainly using flow-through extraction from the DBS card. He then proceeded to introduce his own procedure to produce a cell-disintegrated blood (CDB), which provides a clear sample containing no cell debris. The procedure is as follows: First, a sedimented blood sample is remixed by vortexing and spiked with an appropriate internal standard dissolved in dimethyl sulfoxide (DMSO). Then, the sample is perfused through a heated (LC) capillary where residual blood cells are eliminated by this heat-shock treatment. The output can be coupled on-line to an SPE-LC–MS-MS setup for final analysis. An alternative approach is to snap-freeze the blood sample in liquid nitrogen, which accomplishes the same goal. An actual example of the direct analysis of cyclosporin A in whole blood was shown. The advantages of the CDB approach include no need for an accurate blood volume, addition of internal standard to the native sample, and blood volumes of less than 10 µL, as well as time and consumable saving.
H.K. Lee of the National University of Singapore has been an advocate of solvent-minimized sample preparation and has been a longtime investigator of the use of membranes in sample isolation. In his oral presentation, Lee summarized much of his work in hollow-fiber liquid-phase microextraction, in which a hollow-fiber membrane is used as an interface between the donor and acceptor phases to give an added degree of selectivity. He showed examples in the areas of environmental and biological applications.
Detection Techniques
By perusing the abstracts, I tabulated the detection principles (Table VIII) that were used on a relative basis in the various presentations at HPLC 2012. Not every abstract indicated the detector that was used so only those that provided this information were counted. The category assignments were based on the main emphasis of a particular scientific paper as well as separation and detection techniques used. Again, MS clearly dominates the detection category. If one adds up the use of MS and tandem MS in chromatography and electrophoretic techniques, 47% of the papers presented at HPLC 2012 used this detection technique. Compared to last year (1), there was a slight decrease in the category. Despite the higher cost, the remarkable selectivity and sensitivity of both the single-quadrupole and MS-MS techniques such as quadrupole time-of-flight (QTOF) and triple-quadrupole MS are favored by chromatographers and mass spectrometrists alike.
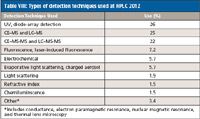
Table VIII: Types of detection techniques used at HPLC 2012
The MS theme permeated many of the talks not only as a detection method for LC and CE, but as an invaluable tool in compound identification and quantification especially for biomolecules at trace levels. One of the plenary lectures by Richard Smith of Pacific Northwest Laboratory in Richland, Washington, discussed the great strides that the combination of HPLC and MS made in his laboratory, devoted to biomedical research and translational applications. A lot of the work in his laboratory deals with nanoscale separations (such as very low flow rates that provide high electrospray ionization efficiencies, proteomics measurements for biomarker discovery and verification, and high-throughput applications). In addition, they use both 1D (better quantitation, faster measurements) and 2D (when better coverage of the proteome is needed) separations but the key is the continued pushing of MS measurements using techniques such as electrodynamic ion funnels that enhance ion production and transfer efficiencies and ultrasensitive and selective targeted multiple reaction monitoring. Smith's story was echoed by the many oral and poster presentations using LC– and CE–MS and MS-MS measurements, especially in the "omics" categories.
This year a very strong showing of UV detection, especially diode-array detection, was the favored detection technique, mostly in the applications and column evaluation examples. Fluorescence detection, evaporative light scattering and charged aerosol detection, and electrochemical detection all showed a slight growth, but these and other detection techniques reported in Table VIII are used infrequently.
HPLC 2013 (Europe) and 2013 (Australia) Are Next
The next major Symposium in this series, the 39th International Symposium on High Performance Liquid Phase Separations and Related Techniques (HPLC 2013), will be held for the second time in Amsterdam, The Netherlands, on June 16–20, 2013. The chairman of the upcoming event is Professor Peter Schoenmakers, of the University of Amsterdam. For more information consult the official website: www.hplc2013.org. The 40th Symposium will also be held in 2013 in Hobart, Tasmania, Australia, on November 18–21. The Chairman of this meeting is Paul Haddad. For more information go to www.hplc2013-hobart.org. Bookmark this website so that you can keep up on the latest happenings.
Acknowledgments
For personal reasons, I was unable to attend HPLC 2012 myself and relied on the notes and comments of others for this article. I would like to acknowledge the contributions of my Agilent colleagues from Wilmington, Delaware, who supplied notes on some of the sessions: Xiaoli Wang, Jim Martosella, Michael Woodman, and Gerard Rozing. A very special thanks goes to Maureen Joseph, also of Agilent, who took very copious and thorough notes on many of the column sessions. Also, I would like to thank Dr. Astrid Gjelstad, of the University of Oslo in Oslo, Norway, who provided notes on some of the sample preparation sessions. Finally, I would like to thank Renee Olson from the CASSS organization for providing some of the meeting statistics.
References
(1) R.E. Majors, LCGC North Am. 29(9), 802–816 (2011).
(2) W.K. Way and C.T. Santasania, Chromatography Today 5(2), 12 May/June (2012).
(3) R.E. Majors, LCGC North Am. 30(8), 626–633 (2012).
Ronald E. Majors

Ronald E. Majors
"Column Watch" Editor Ronald E. Majors is Senior Scientist, Columns and Supplies Division, Agilent Technologies, Wilmington, Delaware, and is a member of LCGC's editorial advisory board. Direct correspondence about this column via e-mail to lcgcedit@lcgcmag.com.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








