Highlights of HPLC 2009
LCGC North America
Ron Majors provides first-hand coverage of some of the technology and application advances reported at HPLC 2009.
The 34th International Symposium on High Performance Liquid Phase Separations and Related Techniques, which alternates between Europe, Japan, and North America, was held, for the first time, in Dresden, Germany from June 28 to July 2, 2009. More affectionately known as HPLC 2009, the symposium is the premier scientific event for bringing together the myriad of techniques related to separations in liquid and supercritical fluid media. Chaired by Prof. Christian Huber of the University of Salzburg, Austria, HPLC 2009 assembled 1240 scientists from a total of 51 countries. This number included 270 vendor representatives from over 64 exhibitors for the three-day instrument, software, and consumables exhibition. Students constituted nearly a quarter of the conferees, which speaks highly for the next generation of separation scientists. Based upon the number of attendees and exhibitors, the worldwide economic crisis did not play heavily into the support for this important conference.

Ronald E. Majors
The five-day plus event had a total of 128 oral presentations in plenary and parallel sessions and over 600 posters in sessions with 20 themes. With an ample social event schedule, 15 vendor workshops (some with free lunch), 12 tutorial educational sessions, and eight short courses, the latter held during the previous weekend, attendees had their hands full deciding how to allocate their time. The tutorials were particularly well attended and covered current topics such as bioanalytical liquid chromatography (LC)–mass spectrometry (MS)-MS, miniaturization, validation, biomarkers, stationary phases, quality by design, speciation, multidimensional LC, microchips, and hyphenated techniques.
Trends in Liquid-Phase Technology and Techniques
Obviously, high performance liquid chromatography (HPLC) was the predominant technology in the technical sessions at the symposium but increased use of electrophoretic techniques, mostly in a capillary format, was strongly evident. From a perusal of the poster and oral presentation abstracts, I broke down some of the major areas of coverage in this year's symposium. These tables are useful to spot trends in the technology, applications of liquid-phase, and detection usage. Many of the topics that are currently "hot" in the separations sciences were introduced in this series.
Table I provides a rough breakdown of the coverage of liquid-phase technology and techniques in the separation sciences. Compared with HPLC 2008 (1,2), some slight shifts in technology emphasis were noted. Although column technology always leads the pack, this year, about a quarter of the columns' papers dealt with monoliths, down from a third last year, but with more emphasis on polymeric-based monoliths that have less intelligential property protection compared with silica-based monoliths. Papers dealing with polymeric monoliths outnumbered papers on silica monoliths about 4:1. One exciting new development in polymeric monoliths was those that can separate small molecules; originally the small molecule domain was for the silica-based monoliths only. Three other "hot" areas in column technology this year were the continued interest in sub-2-μm porous packings coupled with ultrahigh-pressure liquid chromatography (UHPLC) instruments; the explosive attention in the technique of hydrophilic interaction liquid chromatography (HILIC) for the separation of polar analytes (23% of all LC column papers touched on this subject); and the new breed of superficially porous packings (also referred to as pellicular, porous layer beads, and fused-core packings) that are said to rival the sub-2-μm particles in terms of column efficiency but with substantially lower pressure drops. A very hot but rather specialized topic was the use multidimensional chromatography — coupled columns, column switching, and the like. Especially strong was the coverage of comprehensive LC×LC, a subject of two thirds of the multidimensional chromatography papers. With chromatographers encountering more complex samples, sometimes with thousands of compounds present, these multidimensional techniques are about the only way to tackle such mixtures.

Table I: HPLC 2009 papers presented by technology or technique
Sample preparation technology was well represented in the poster papers. Most prominent was solid-phase extraction (SPE) in its various formats: cartridges, pipette tips, 96-well plates, syringe-packed sorbent, as well as selective phases such as molecularly imprinted polymers (MIPs), immunoaffinity phases, restricted-access media, and mixed-mode phases. Liquid-phase microextraction via hollow fibers, electromembrane extraction, and protein precipitation plates with built-in phospholipid removal were new areas getting attention.
Even though there have been reports of its demise, electrodriven separation techniques — for example, capillary electrophoresis (CE), capillary zone electrophoresis (CZE), micellar electrokinetic chromatography (MEKC), and isoelectric focusing (IEF) — had a strong representation again this year. Recent introductions of improved CE instrumentation on the market could perhaps bring new life into this technique widely used for inorganic anions and cations, enantiomeric compounds, proteins, and drug-stability measurements. A continued lack of interest in capillary electrochromatography (CEC) was noted with only eight presentations at HPLC 2009. Only a few years ago, lecture rooms on CEC topics spilled out into the hallway and the technology was to replace both HPLC and CE. However, the "killer" application for the technique was never found; any separation performed by CEC could be done by gas chromatography (GC), SFC, or LC without all the associated problems.
Areas of Application
Table II is a breakdown of the most popular application areas reported at HPLC 2009. Although the number of oral presentations on proteomics, biomarkers, protein separations and identification still leads the way, HPLC finds widespread use in the analysis of drugs and endogenous compounds in biofluids and tissues in pharmaceutical and clinical testing. The strongest area of growth in the past year has been the application of liquid-phase techniques in foods, beverages, and food safety. This increased attention to the safety of our food supply is no doubt a result of reports on toxic food contaminants making the news in the past year or so.
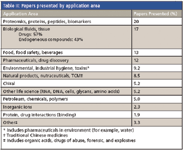
Table II: Papers presented by application area
In this installment of "Column Watch," I will present some of the scientific highlights of HPLC 2009. This report also will cover the various awards and honorary sessions that took place. Because it was virtually impossible for one person to cover all oral presentations and posters adequately, my coverage will somewhat reflect a personal bias, although I was able to get presentation notes from some of my colleagues who are acknowledged at the end of this column.
Awards and Honors at HPLC 2009
Horvath Award: For the fourth year in a row, the Horvath Award sessions, named for the late Prof. Csaba Horvath, one of the founders of this series and a mentor of young scientists, were featured. This award, supported by HPLC, Inc., a nonprofit group under the guidance of the Permanent Scientific Committee, was established for young scientists in the separation sciences under the age of 35. The award, based upon the best oral lecture presented in the Horvath Sessions, was selected by a jury named by the Permanent Scientific Committee and consists of a cash prize, invitation to present an oral at HPLC 2010, and a trophy. This year's winner was Andre de Villiers from Stellenbosch University in South Africa. His coauthors were F. Lynen and P. Sandra from Ghent University in Belgium. The title of the award-winning presentation was "The Effect of Analyte Properties on the Kinetic Performance of Liquid Chromatography Separations." The researchers showed that the analyte properties affect the kinetic performance of a chromatographic system. Often, columns are measured with standard test compounds that are different from those being analyzed in "real life." These authors showed that the optimal particle size–maximum pressure combination depends upon the analyte under investigation. Noteworthy differences in kinetic performance between pharmaceutical compounds and test compounds highlighted the importance of using real sample to perform these evaluations. They also found that pH of the mobile phase also plays a crucial role in kinetic performance data.
Poster Sessions and Best Poster Awards: The mainstay of HPLC 2009 was the poster sessions, where more detailed applications and methodology studies were reported, often in very specific areas, and face-to-face discussions with the authors were conducted. Fortunately, many of the poster authors were kind enough to provide small reproductions of their poster papers that could be taken for later perusal. Some authors collected business cards and addresses for sending poster reprints by mail or e-mail. Compared with HPLC 2008 (1), the number of posters was 35% higher. The posters were split into two two-day sessions; by staggering the days that the posters were staffed by presenters, the sessions did not appear to be crowded. From walking around the poster areas, there appeared to be quite a number of "no shows," often from third world countries, who apparently did not get funding to attend or merely wanted their abstracts to appear in the program.
The Poster Committee co-chairs at HPLC 2009 were Prof. Peter Schoenmakers, a member of the Permanent Scientific Committee from the University of Amsterdam, The Netherlands and Dr. Gerard Rozing, Agilent Technologies, Waldbronn, Germany. The 42 members of the Poster Committee devoted a great deal of time and worked very hard to narrow down the collection of posters to 15 by the end of the third day and then helped to select the three winners by the Thursday afternoon of the symposium. The selection criteria were based upon three factors: inspiration, transparency, and presentation, and winning posters were viewed by a large number of committee jurors.
The Best Poster Awards, sponsored by Agilent Technologies (Wilmington, Delaware), were announced at the Closing Session. After narrowing the field down to 15 posters, the three winners selected as the top vote-getters received cash prizes and a certificate commemorating their achievement. This year's winning poster was entitled "Quantitative Three-Dimensional Structure-Transport Analysis in Chromatographic Beds of Arbitrary Cross-Section" by Siarhei Khirevich along with co-authors A. Holtzel, D. Hlushkou, U. Tallarek, and A. Morgenstern-Seidel of the University of Marburg, Marburg, Germany. These investigations involved the use of numerical analysis methods to determine hydrodynamic dispersion in various configurations of spherical particle packed beds (circular, semicircular, quadratic, rectangular, and trapezoidal cross sections) in LC. The authors concluded from these simulations that noncylindrical geometries can significantly increase dispersion. As might be expected, higher packing densities reduce dispersion. The efficiency of trapezoidal cross-sections can be optimized by considering the lateral dimensions of a conduit. These observations might have a strong impact on the design of microfluidic-based columns in the future. A picture of Siarhei holding his 1st prize certificate is shown in Figure 1a.

Figure 1: Photos of the top three winners of the Best Poster Awards at HPLC 2009: (a) First place: Siarhei Khirevich of the University of Marburg, Germany; (b) second place: Isabelle Francois of Exxon-Mobil, Machelen, Belgium; and (c) third place: Melanie Haugg of the University of Ulm, Germany. Photos by PHOTO:GRYSA, Braunschweig, Germany.
The second place winner of the Best Poster contest was to Isabelle Francois of Exxon Mobil, Machelen, Belgium, along with co-authors A. dos Santos-Pereira and P. Sandra of the University of Ghent, Belgium. Their work was entitled "Comprehensive and Off-line Supercritical Fluid Chromatography-Reversed Phase Liquid Chromatography for the Analysis of Complex Triglyceride Profiles." Using a 2D setup, their first dimension was a 50-cm-long silver-ion loaded column operated with a supercritical fluid mobile phase and the second dimension was a 10-cm C18 monolith or a 45-cm-long C18 column packed with sub-2-μm particles and operated with a nonaqueous reversed-phase mobile phase. They compared the two systems by using on-line and off-line conditions and used the peak capacity of each as a measure of effectiveness. For a very complex fish oil sample, they found that off-line gave more information (higher peak capacity) but took a longer time than conducting the experiment on-line. Francois' picture is shown in Figure 1b.
The third place winner of the Best Poster award went to another German research group from Ulm University. The first author was Melanie Haugg and her co-author was T. Welsch. The title of their poster was "Star-like Poly(alkyleneoxide) Coated Open Tubular (OT) Columns for Tuning the Separation Mode in Liquid–Liquid Chromatography." Although capillary columns routinely are used in GC, they have not received widespread use in HPLC. Using very tiny open tubes of 5 and 25 μm in internal diameter, these authors coated a polymeric material that possessed both polar groups (like diol, ether, urethane, and amino) and nonpolar groups (alkyl chains). The hydrophobic backbone could be finetuned by the proper choice of monomer. These OT columns could be used for reversed-phase, HILIC, or normal-phase separations. The columns were run in both pressure- and electroosmotic-driven flow modes and provided excellent performance. A photo of Melanie holding her third place certificate is depicted in Figure 1c.
This year, Best Poster Awards for "Innovation in Pharmaceutical Analysis" were presented by Pfizer. A total of six awards were given during the Closing Ceremony. The top pharmaceutical poster was presented by Raul Nicoli of the School of Pharmaceutical Sciences, University of Geneva, Switzerland. His co-authors were R. Curcio, S. Rudaz, and J.-L. Veuthey of the same institution. Their poster was titled "Development of an In-capillary Method to Nanoscale Automated in vitro Cytochrom P450 (CYP450) Assay." In vitro CYP450 assays are important in the pharmaceutical industry for understanding the oxidative phase 1 metabolism of drugs. In their work, they developed a novel method using a capillary as a reaction vessel, which consumed only a few nanoliters of enzyme solution (100–500 times less than classical experiments). Their strategy decouples the in-capillary enzymatic assay from the analysis of metabolites and substrates and is performed off-line by UHPLC coupled to MS using a ballistic gradient; the separation time is less than a minute. They successfully applied their approach to six CYP450 isozymes of known metabolic pathways.
Opening Session and Awards
Opening Ceremony plenary lectures, presented on the first evening of the Symposium, are supposed to be leading edge, thought-provoking presentations that are to inspire attendees to think beyond the box. After a fitting Opening Ceremony involving Welcoming Remarks by Prof. Huber followed by the Dresden Brass Quintet, a five-piece ensemble that entertained the large crowd of chromatographers, various prizes were announced including the Martin Gold Medal, awarded to Prof. Wolfgang Lindner of the University of Vienna, Austria, who unfortunately could not attend due to a last minute broken shoulder. Prof. Lindner is recognized widely for his contributions to the separations of enantiomeric compounds. This prize is presented by The Chromatographic Society, which is based in the U.K. with international connections and was created for the promotion of and development of separation science. In 1978, Prof. A.J.P. Martin gave permission for his name to be associated with the "Martin Gold Medal." The Martin Gold Medal is awarded to scientists who have made outstanding contributions to the advancement of separation science. The Chromatographic Society also awarded its Jubilee Silver Medal to Prof. Gert Desmet of the Free University of Brussels, Belgium for his fundamental studies on flow modeling effects and kinetic plot investigations for comparison of HPLC column performance. The Jubilee Medal is awarded to up-and-coming scientists who have made major use of separation science or to scientists who have made meritorious contributions to a particular area of separation science.
Finally, the Widmer Award, sponsored by Novartis Pharmaceuticals along with the Analytical Chemistry Division of the Swiss Chemical Society, was presented to Prof. Gunter Fuhr, Director of the Fraunhofer Institute for Biomedical Engineering (IBMT) in St. Ingbert, Germany for his work in biotechnology research, single-cell separations, and biochips. The late Michael Widmer was formerly head of the research-focused central analytical unit in Ciba, Basel, Switzerland and one of the old grand analytical chemists in the 1980s and 1990s. The Michael Widmer Award is granted to an individual who had demonstrated extraordinary performance in analytical sciences.
Plenary Lectures
This year, three notables gave plenary lectures on Sunday evening. The first lecturer, well known to chromatographers, was Prof. Pat Sandra of the University of Ghent. His title was "Present State-of-the-Art and Future Challenges of Fluid-based Separations for the Pharmaceutical and Chemical Industries." Pat has contributed to many areas of chromatography including GC, supercritical fluid chromatography (SFC), and HPLC. His lecture highlighted fluidic separation methods, especially those interfaceable to MS. At one time, mass spectroscopists thought that chromatography was not really needed and MS would solve all the problems. The current thinking is that present-day chromatography makes better MS. Surprisingly, chromatographers are aiming for extremely high plate counts but, as Pat pointed out, most often, "real world" separations need only 5000–10,000 plates, although the increasing use of UHPLC to improve throughput can be very convincing. The use of increased temperature can lead to an increase of a factor of two in separation speed. He feels that the use of kinetic plots is currently the optimum way to compare columns and showed an example of the use of 12 columns in series packed with 5-μm particles gave better overall performance (peak capacity of 1230) than smaller particle columns but at the expense of time, all predicted by comparison of kinetic plots of 1.8-, 3.5-, and 5.0-μm columns. Future challenges facing chromatography laboratories include the management of knowledge and know-how. Many of the traditionally trained chromatographers are retiring and the newer scientists now doing separations need to be educated in the fundamentals of chromatography and MS. "Green" analytical techniques such as SFC and alternatives to current hazardous organic solvents need to be investigated. Pure water as a mobile phase with silica columns might be one approach to cut the use of organics. The reduction of column internal diameter is another approach that should become standard, especially as the heat generated in wider bore columns in UHPLC can cause a loss of resolution. Quality by design also will be used more and more in the development of rugged chromatographic methods.
The next plenary lecture was presented by Thomas Hankemeier of Leiden University, The Netherlands. His theme was metabolomics-driven systems biology, a frequent theme of previous plenary lectures in this series. A systems-based approach to human diseases, rather than a target-based approach, should ultimately be a more cost-effective way to provide the right treatment for the patient. However, this approach requires good quality data on a wide range of metabolites and proteins. Through the use of high performance LC–MS, many strides have been made in this direction but major challenges still remain due to the needs for improved sensitivity over a wide dynamic range, better quantitation, and metabolite identification. Ion suppression in MS is a particularly difficult problem, especially for reversed-phase separations that are often unsuitable for many polar metabolites. Their laboratory is investigating HILIC using high concentrations of acetonitrile in the mobile phases and a polymeric amino column. Samples frequently are desalted before HILIC by using an ion-exchange column. Plasma lipids are another source of difficulty and some of the newer lipid-removal columns can be particularly useful here. Great strides are being made in the systems biology area and with a number of laboratories are working together to further the science, the outlook appears to be bright.
The final speaker in the opening session was another chromatography expert, Prof. Ed Yeung of Iowa State University, Ames, Iowa. Prof. Yeung has been directing his attention to the study of the dynamic behavior of single DNA molecules at chromatographic surfaces and dazzled everyone with his pictures of individual molecules using total internal reflectance fluorescence microscopy to study intermolecular interactions. Real-time molecular motion at the surface was recorded to reveal adsorption behavior and conformational dynamics of probe DNAs with "sticky" ends of different numbers of unpaired bases. Interesting modifications of the DNA using amine, alcohol, or acid functionalities allowed one to observe different chromatographic interactions with the surface. However, protein studies using this approach appear to be more challenging because adsorption can be very much influenced by pH, which affects internal changes in the protein as well as charges on the surface of the chromatographic media. Exclusion effects in pore structures also can occur; studies on size and shape effects can lead to a better understanding of protein interactions. Quoting Chairman Huber, "For me, this was the first time to see with my own eyes that we can now start to study the behavior of single molecules in a dynamic chromatographic system."
New Column Technology Highlights
As seen in Table I and similar to the last seven years of HPLC Symposia coverage (1–12), the development and study of columns and stationary phases still dominates new technologies. Both oral sessions and poster sessions were devoted to column technology, retention mechanisms, high-throughput application, UHPLC, and the like. If one combines all papers pertaining to column technology, about a third of the presentations at HPLC 2009 covered this topic. Despite all the advances made in column technology to date, investigations on further developments in academia as well as the commercial side are still taking place.
Small porous particles and superficially porous particles: From what I observed at HPLC 2009, one of the current "hot topics" in HPLC column technology was the discussion of the alternative approaches for developing faster separations and generating more column efficiency at lower pressure drop and which approach will be favored in the long run. A most interesting lecture on this topic was presented by Xiaoli Wang of Astra Zeneca Pharmaceuticals, Wilmington, Delaware in the pharmaceutical analysis session. In his lecture, Wang compared the performances of sub-2-μm particles and superficially porous particles (2.7 μm) under optimized UHPLC conditions (column length and flow rate). Using the Poppe plot technique, he and his co-authors Y. Zhang and P. Mukherjee conducted flow studies to compare the kinetic characteristics of the two different particles. They found that for very short analysis times (for example, < 1 min), similar performance can be obtained on both stationary phases but as the analysis time increases, the superficially porous particles started to outperform the sub-2-μm particles and much higher plate counts (>2X) can be obtained at very long analysis times (for example, >3 h). Experimental observations using isocratic conditions agreed with the calculated results. He went on to further discuss gradient elution results in terms of peak capacity of both phases and the same trend was observed.
The Desmet group from the Free University of Brussels, Belgium also have looked at the porous, superficially porous particles and monoliths using their kinetic plot approach. Kinetic plots are employed to predict the performance potential of different column types and particle sizes under a variety of temperature and pressure conditions. Deirdre Cabooter of the Brussels group presented a paper on the use of the kinetic plot to design single and coupled column systems. After a clear explanation of how to transfer a van Deemter plot to a kinetic plot, she went on to show that using the fixed-length variant of the kinetic plot method, an analysis performed near the optimal flow rate on a column could be done 200% faster by switching to a longer column and operating at a higher pressure. Another example of comparing a monolith to a porous particle column showed that the monolith column was better only when the number of plates required exceeded 200,000. Ms. Cabooter cautioned the audience that when working at very high pressures, some of the basic assumptions of the kinetic plot method such as the independency of the plate height and retention factor on the column length or when the k value changes with pressures above 1000 bar, might not hold true. Likewise, caution should be exercised when a column is run under nonadiabatic conditions such as elevated temperatures obtained by using a forced-air oven, when extracolumn effects might be significant, or if an axial change in the bed structure occurs.
Most kinetic plots to date have been generated with isocratic separations in mind but at HPLC 2009, Monika Dittman of Agilent Technologies, Waldbronn, Germany and coauthors extended the kinetic plot model to the optimization of gradient separations as a function of column performance and compound retention parameters. In addition, the model was used to investigate the influence of extracolumn contribution, in particular the influence of connection capillary diameter, on kinetic performance.
Monoliths: Monolith columns have been desirable because they exhibit high permeability–low pressure drop (due to increased bed porosity), show good separation efficiency, have the absence of frits to confine the packing material, are easy to fabricate, and can be made fairly reproducibly. In his Keynote Lecture in a monolith session, Frantisek Svec of Lawrence Berkeley National Laboratory, Berkeley, California gave an excellent overview of the technology and mentioned that monoliths have seen applications in a wide variety of separation sciences (for example, HPLC, SPE, GC, thin-layer chromatography, and microfluidics) and beyond (combichem supports, high-throughput bioreactors, electrochemistry). Although this technology has been around for several years, as a routine tool it has yet to see widespread acceptance on the commercial side but improvements continue to be made for both polymer- and silica-based monoliths. In the past, overall observations indicated that silica-based monoliths seem to work best for small molecules while the polymeric monoliths are best for macromolecules. New reports at HPLC 2009 showed that polymeric materials can be synthesized that have better performance for small molecules.
During his lecture, Svec talked about the new generation of high surface area "hyperlinked" polymeric monoliths that should be more useful for small molecule separations. Later in the same session, Greider Andreas and coauthors from the Leopold-Franzens University in Innsbruck, Austria gave a paper on the influence of polymerization time on the porous properties of organic monolithic stationary phases. By methodically studying typical thermally initiated free radical polymerization parameters (for example, porogen-to-crosslinker ratio, porogen nature and composition, polymerization temperature, and polymerization time), he and his research group found that resulting monoliths could be optimized for the separation of small molecules or large biomolecules. With very short reaction time, a biomodal pore size distribution is observed, similar to what happens when silica monoliths are polymerized from during the sol gel process. The polymeric monolith phases showed high mechanical robustness and low swelling in organic solvents due to their high level of crosslinking.
Emily Hilder of the University of Tasmania, Australia presented a paper on improving the performance of polymeric monoliths by combining some of the Dionex technology of coating agglomerated latex charged ion-exchange nanoparticles onto the surface of an oppositely charged monolith and using this material to separate small ions as well as large biomolecules. The thin layer of nanoparticles results in rapid mass transfer and therefore high separation efficiency up to 50,000 plates/m. These polymeric monoliths can be used at high temperatures (~140 °C) for further efficiency improvement, although the PEEK column hardware might have some problems due to a glass transition occurring at high temperatures. The columns also can be used with pure water as a mobile phase and can be combined with temperature programming.
In the second monolith session, Nobuo Tanaka and coworkers, Kyoto Institute of Technology, Kyoto, Japan, further described their work on high-efficiency monolithic silica capillary columns for microLC. They prepared columns using the sol gel reaction of tetramethoxysilane (TMOS) and methytrimethoxysilane (MTMS) accompanied by phase separation in the presence of polyethyleneglycol. The incorporation of the methyl group into the hybrid monolith contributed to its retention. They studied the ratio of TMOS to MTMS and investigated the effects on both permeability and efficiency, always needing a balance between the highly efficient mesoporous skeletons and macropores that influence permeability. They found that a starting ratio of 9:2 TMOS/MTMS gave the highest permeability and good efficiency. A 1.5-m capillary column (consisting of capillaries joined in series) gave 1.5 million theoretical plates, allowing the separation of benzene from deuterated benzene. The column showed efficiency equivalent to a 2.2–2.3 μm microparticulate column with the pressure drop comparable to a 5-μm column. The efficiency was not quite as high as a monolith prepared entirely from TMOS but columns were easier to prepare. To get higher efficiency, a higher degree of homogeneity will be required but the pressure drop can increase near that of a 3-μm particle column of the same dimensions.
In addition, the Tanaka group was able to modify these phases by octadecylsilylation using N,N-diethylaminodimethyloctadecylsilane or by the copolymerization of octadecyl methacrylate (ODM) with anchor (methacryloxypropyl) groups on the monolithic silane. The ODM phase showed comparable efficiency to the octadecylsilane phase but showed greater dispersion interactions, giving rise to selectivity differences for molecules having halogen atoms and sterically-hindered isomers.
On the commercial side of silica monoliths, Karen Cabrera of Merck KGaA, Darmstadt, Germany reported on their work based upon some earlier findings of Tanaka. The conventional Chromolith columns with a defined bimodal structure (macro- and mesopores) show a separation efficiency equivalent to a 3.5-μm particle packed column and a column backpressure comparable to that of a 10–12 μm particle packed column. Column performance of monoliths is related to the domain size (sum of macropore and skeleton size) of the silica network as well as other parameters such as mesopore size and radial–axial homogeneity. The performance of the silica monoliths is governed by the "A" term determined mainly by the macropore size. Merck chemists prepared six columns with different macropore sizes and clad the columns in PEEK polymer. The columns were derivatized with ODS and endcapped. The resulting monoliths showed a decrease in the domain size from 3.28 μm (macropore/skeleton size: 2.0/1.3 μm) down to 1.6 μm with a corresponding decrease in plate height down to 7.4 μm. A further decrease to 1.1 μm gave no further decrease in plate height. The best columns manufactured so far show 257,000 plates/m with a corresponding plate height of 3.8 μm. Further works will be performed to improve the radial heterogeneity and achieve better control of the phase separation during the sol gel process.
A paper by Fabrice and Guiochon of the University of Tennessee, Knoxville, Tennessee and Oak Ridge National Laboratory, Oak Ridge, Tennessee discussed mass transfer in monolithic column with different pore sizes and they concluded that to improve the "A" term of silica monoliths, a number of properties need to be improved: a more ordered 3D structure; a narrower through-pore size distribution, and a decrease in radial flow homogeneity, in agreement with the Merck people.
Little by little, the monolithic columns are improving as researchers optimize the domain sizes of both polymeric and silica monoliths. It will be interesting to see if these improved monoliths are commercialized and become more commonly used in the industry.
HILIC: Similar to last year (1) and as can been seen in Table I, HILIC received a great deal of attention at HPLC 2009. HILIC is a separation technique for highly polar analytes that gets around some of the problems associated with reversed-phase chromatography such as low retention or phase collapse (dewetting). HILIC uses a polar stationary phase such as bare silica gel or polar-bonded phase (for example, diol) and requires a high percentage of a nonpolar mobile phase, similar to the requirements for normal-phase chromatography. However, unlike normal phase, which uses nonpolar solvents like hexane and methylene chloride and tries to exclude water from the mobile phase, HILIC requires some water in the mobile phase to maintain a stagnant enriched water layer on the packing surface into which analytes can partition selectively. In addition, water-miscible organic solvents are used. Under HILIC, polar analytes are well retained and are eluted in order of increasing hydrophilicity. The high organic solvent content of the mobile phase is especially attractive for LC–MS because ionization efficiency is improved and suppression effects reduced. The use of a high percentage of organic solvents also means that the viscosity is lower than that of a typical reversed-phase mobile phase. Thus, the pressure drop is lower and the efficiency might be higher.
Uwe Neue of Waters, Milford, Massachusetts presented a kickoff lecture in a columns session where he discussed the mechanism (both partitioning and ion exchange can occur), advantages and pertinent equations governing retention for HILIC in the separation of polar compounds. He mentioned that the first reported HILIC separation was published by Fred Rabel in 1976 (13) when he was working for Whatman in Clifton, New Jersey. He and his coauthors used an amino bonded phase column to separate carbohydrates using a water–acetonitrile mobile phase. As the percentage of water increased, retention times were decreased, indicative of the HILIC mechanism, although it was not called HILIC in those days. In Neue's presentation, he introduced new sub-2-μm HILIC phases based upon amide functionality that provides a different selectivity relative to silica gel–based HILIC columns. In addition, the amide group does not form Schiff bases unlike a typical amino column. Some applications of HILIC to oligosaccharides and glycans were demonstrated. An interesting observation was the influence of pressure on HILIC retention for some 2-aminobenzamide–derivatized N-linked glycans from human immunoglobins. Retention times decreased with as the pressure increased. He attributed the pressure effects to changes in the analyte volume due to compressibility of the hydration sphere. Both fluorescence of the derivatized glycans and evaporative light scattering detection were used in the applications.
Multidimensional and Comprehensive Liquid-Phase Separations
As samples become more complex, even with the best and most efficient columns available, the peak capacity of a single column is no longer sufficient to resolve all the peaks to baseline. Peak capacities of 1000 can be achieved with a single column in a very long analysis time but to handle samples with thousands and tens of thousands of components, multiple columns with different separation mechanisms are needed. The total peak capacity of a multidimensional system is equivalent to the peak capacity of the initial column times the peak capacity of the second column. This statement is true only if the two chromatographic modes are orthogonal — totally different mechanisms of separation.
Multidimensional chromatography has been around for many years but has achieved minimal success. With high-speed switching valves controlled by computerized systems, techniques such as heartcutting and flow diversion to eliminate uninteresting peaks are getting more commonplace. Comprehensive LC, termed LC×LC, is a technique that attracted the most interest at HPLC 2009. In this approach, every single fraction from the first dimension is directed to a second dimension to be further separated and identified. There can be off-line and on-line approaches to address this coupling. In the on-line LC×LC approach, switching is accomplished without shutting down the flow of the first dimension column by storing the effluent from column one in a loop or packed bed and switching that storage device onto the second column once it has finished its analysis. The implication here is that the second dimension must be extremely fast in the time scale of the storage of entire effluent from column one.
A short course, two oral sessions, scattered oral presentations, and many posters were devoted to comprehensive and multidimensional techniques.
In a keynote lecture opening a session on multidimensional separations and column coupling, Prof. Georges Guiochon of the Univ. of Tennessee, Knoxville, Tennessee and Oak Ridge National Laboratory, Oak Ridge, Tennessee discussed the various options available to those who need to use multidimensional chromatography to solve their separations problems. On-line 2D separations with all fractions going from column 1 to column 2 gives the optimum time savings but the second dimension must be fast and the observed peak capacity might not be optimum. The "Stop-and-Go" 2D experiment is similar to the on-line approach but the flow from column 1 is stopped while the analysis on column 2 is proceeding until completion. Obviously, this process is slower but might allow better peak capacity because the second dimension also can be slower and allow better overall resolution. The off-line 2D experiment is the slowest but might allow the highest peak capacity because many fractions can be collected. The Murphy-Schure-Foley rule says that fractions should be made with the time equal to the average standard deviation in the first dimension. In the off-line approach, the separation mechanisms can be totally different, giving rise to better orthogonality. In the off-line 2D chromatography, parameters to be optimized together include characteristics of the first and second dimension columns, frequency of fraction collection, and time needed to process a fraction. Because samples analyzed by multidimensional chromatography are usually complex, gradient elution is used commonly. Peak capacity in gradient elution will increase with the theoretical plates of a column and with an increase in gradient time but the total time also is increased with slower gradients. The total cycle time required is the gradient time + dwell time + time of regeneration + injection time. Showing some examples of a 2D experiment, by optimizing the process, Guiochon showed that one can achieve very large total peak capacity within a "reasonable" time. In off-line 2D, a peak capacity of 16,000 could be achieved in 24 hours while in the on-line approach, a peak capacity of 1000 could be achieved in 1 h assuming the two dimensions are orthogonal.
In another paper, Sebastiaan Eeltink and coworkers from Dionex, Amsterdam, The Netherlands compared the 1D and 2D-LC separation performance for peptide digests using poly(styrene–divinylbenzene) (PS-DVB) monolithic capillary columns. The LC conditions and column technology were optimized to maximize the peak-capacity-to-analysis-time ratio. Short 50-mm-long monoliths yielded better peptide separations than the 250-mm-long monolithic column for gradient times below 60 min, which was related to the macropore size. A peak capacity exceeding 1000 was recorded on a 1-m-long monolith applying a 600-min gradient. In the off-line 2D-LC setup a large-internal-diameter 1D column was applied providing high sample capacity and a small-internal-diameter column applied as 2D column provided high mass sensitivity and easy coupling with MS. The number of fraction collected in the first dimension and analyzed on the 2D column was optimized towards sample complexity. For example, they were able to obtain a peak capacity of 2400 with a 164-min gradient.
Later papers gave practical examples of comprehensive multidimensional chromatography. In his keynote lecture on Wednesday, Prof. Tyge Greibrokk of the Univeristy of Oslo, Norway gave a nice overview on the advantages and limitations of multidimensional chromatography. He also compared the on-line and the off-line approaches but focused most of his talk on the on-line multidimensional chromatography separations. The rules for successful multidimensional chromatography were stated again:
- True orthogonality.
- Peaks cover the entire separation "space."
- The separation gained in one dimension must not be lost by separation in the second dimension.
He made a special mention that peak capacities often reported in the literature can be exaggerated, especially when calculated from a single peak in a complex chromatogram. He compared the various column and instrument configurations used (for example, narrow internal diameters, short and long columns, high and low speed, trapping columns and loops) matched to applications. He went on to show real world examples of coupling reversed-phase and cation-exchange chromatography for the separation of intact proteins in blood, various first and second dimension column combinations (including strong cation exchange, strong anion exchange, reversed phase, HILIC, and size exclusion) for peptide separations using electrospray ionization (ESI)-MS detection, and for microchip possibilities for proteomics.
Prof. Pavel Jandera of the University of Pardubice, Czech Republic, investigated various combinations of reversed-phase, HILIC and normal-phase columns for the 2D separation of polar compounds. Several of the columns investigated showed dual separation mechanisms. He found that a very polar HILIC first dimension column with an organic-rich mobile phase coupled to a C18 second dimension column operated with a water-rich mobile phase in the reversed-phase mode could be used successfully provided that equilibration time was sufficient for the somewhat noncompatible solvent systems. He also found that, due to dual mechanism characteristics, one column can be used for both reversed-phase and HILIC modes.
Prof. Paola Dugo of the University of Messina, Italy gave a most practical lecture on her group's work on approaches to obtain high peak capacity on real samples using an automated LC×LC system. Using combinations of normal-phase and reversed-phase columns in the first and second dimensions, they were able to resolve complex mixtures of naturally occurring substances. The first dimension column was usually a long column with a small internal diameter that offered high resolving power while the second dimension column provided a very fast separation using a superficially porous particle. Sometimes elevated temperature was used to allow use of longer columns or columns in series. One example shown was the determination of native carotenoids in citrus products. Initially, C18 and C30 columns were tried in a single dimension mode but many compounds were not separated in an orange essential oil sample. So, a 2D separation was attempted using a normal-phase column (cyano, 200 mm × 1.0 mm, 5 μm) in the first dimension and a reversed-phase monolithic column (C18, 100 mm × 4.6 mm) in the second dimension. The normal-phase column did a good job on separating by chemical class while the reversed-phase column separated some of the carotenoid esters. Other examples shown included polyphenols in wine and peptide tryptic digests.
When coupling normal phase using nonpolar organic solvents with reversed phase using aqueous solvents, solvent compatibility becomes an issue. A keynote lecture by Prof. Yafeng, Guan of the Dalian Institute of Chemical Physics, Dalian, Peoples Republic of China and coworkers came up with a novel vacuum-assisted dynamic evaporation loop-type valve interface for 2D LC (normal phase → reversed phase). The interface removes the solvent by dynamic evaporation by vacuum as the solvent enters a fraction loop (10 μL) from the first dimension column and retains the non-evaporable analytes on the wall of the loop. Once the column-switching valve is switched, the mobile phase from the second dimension enters the loop and dissolves the analytes deposited on the wall and carries them to the second dimension column in a compatible solvent. The temperature of the fraction loop can be controlled. The authors studied different evaporation temperatures using samples with different boiling points and investigated sample losses. Not surprising, sample loss depended upon the loop temperature and the boiling point of the compound investigated. For nonvolatile samples, sample loss was negligible. Besides eliminating the problem of solvent exchange, analytes are concentrated in the fractions from the first dimension.
Quite a number of other papers showed great examples of 2D separations of complex samples including xenobiotics in soil, low molecular weight organic acids in food and body fluids, Yerba-mate leaf extracts, phenolics in foods, tryptic digests of human serum albumin, synthetic oligonucleotides, platycosides in natural product extracts, and triacylglycerols in corn oil. Modes that were coupled included nonaqueous reversed phase → argentation (Ag+) chromatography, Ag+ chromatography → reversed phase, normal phase → reversed phase, reversed phase → normal phase, HILIC → reversed phase, HILIC → reversed phase, strong cation exchange → reversed phase, weak anion exchange → nonaqueous reversed phase, SFC → reversed phase, ion pair reversed phase → ion pair reversed phase, reversed phase → reversed phase, CE → CE, and CE → reversed phase. As more commercial instrumentation for 2D LC×LC becomes available, I would expect to see many more applications to complex samples in future HPLC series meetings.
Temperature Studies in HPLC
The role of temperature has been on the list of well discussed topics for the last several years and is driven by the need to reduce column backpressure to more reasonable values, to increase the efficiency of separation, to decrease retention, and by the introduction of column materials and phases that can take higher temperatures up to 200 °C.
At HPLC 2009, more studies on the use of temperature as a separation variable have been conducted. Isothermal temperatures often are used in HPLC to improve mass transfer and lower backpressure. Temperature programming is very often used in GC but seldom in HPLC. Fused-silica capillary columns have a heat transfer efficiency of about 40 times better than conventional stainless steel HPLC columns. So Prof. Fernando Lancas of the University of Sao Paulo, San Carlos, Brazil used packed fused-silica capillary columns with temperature programming in HPLC. Workers from multiple sites of Dow Chemical presented a poster on low thermal mass LC, where resistive wire heating was used to rapidly heat (up to 1800 °C/min) or cool (100–200 °C/min) packed microcolumns (<0.5 mm i.d.) to perform fast temperature programming. When combined with gradient programming, a versatile separation system was developed where cycled temperature programming could be used to fine tune the separation of critical band pairs. Temperature gradient programming also was illustrated in a poster by Prof. Bernd Wenclawiak and coworkers at the University of Siegen, Germany. These workers used temperature programming to separate metal dialkylthiocarbamates more effectively than could be achieved by isothermal conditions. In addition, they were able to cut down on the amount of organic solvent consumption.
Prof. Roger Smith, who will soon be retiring from Loughborough University in the U.K., has long been an advocate for the use of higher temperatures. In his Plenary Lecture in the Pharmaceutical Analysis session, he gave a comprehensive overview on the role of temperature, particularly those above 80 °C, on optimization of HPLC methods. He discussed some of the advantages already covered but stressed the newer column materials such as hybrid phases and coated phases on stable support materials. In addition, because of the reduced viscosity, higher flow rates can be employed. Lesser retention often means that the organic component of the mobile phase can be reduced or even eliminated providing a "greener," less polluting mobile phase. Interestingly, the dielectric constant of pure water at very high (~250 °C) but at subcritical temperatures can be close to that of methanol. Thermal decomposition of pharmaceutical analytes has not proven to be a major problem because the residence time on the column is often only a few minutes. Another poster by a group from Ghent University in Belgium showed that loadability in preparative chromatography can be increased at high temperatures and higher efficiency can lead to increasing peak purity.
An interesting aspect of the use of temperature in HPLC has been the development of temperature-responsive polymers as stationary phases. Temperature-responsive polymers undergo reversible phase transitions that change the microstructure with a change in temperature. The group of Prof. Tadashi Nishio of the Physical Pharmaceutical Chemistry Department at Keio University in Tokyo, Japan has developed such polymers based upon the copolymerization of N-isopropylacrylamide and butylmethacrylate. This copolymer undergoes a reversible phase transition from hydrophilic to hydrophobic that is triggered by a change in temperature. With this chromatographic column, it is possible to use an aqueous phase only for the mobile phase. Retention time can be adjusted by a change in temperature. One of their posters at HPLC 2009 showed that besides small molecules, proteins can be separated under mild conditions and their biological activity can be maintained. Another poster paper coauthored by a group from the Max-Planck-Institute for Colloids- and Interfaces and from the Fraunhofer Institute for Applied Polymer Research located in Golm, Germany, synthesized several thermo-responsive stationary phases by grafting various copolymers onto preformed stationary phases and then used these phases to separate steroids and peptides in pure aqueous environments.
On the "negative" side of temperature effects in HPLC is the frictional heating that results when conventional HPLC columns are run at high flow rates and high operating pressures, especially with sub-2-μm particles. As the mobile phase passes through the particles in the column, heat is generated inside the column due to the impact of viscous forces. A group from the Free University of Brussels, Belgium, and Agilent Technologies, Waldbronn, Germany studied this phenomenon and reported the results in a poster paper. Below 1000 bar, this generated heat does not significantly reduce the column efficiency for columns with no or limited radial heat transfer from column bed to wall (columns under nearly adiabatic conditions). However, in this mode there is a significant temperature effect (up to 20 °C) in the longitudinal direction. In the isocratic mode, a steady state is reached after 20 min or so but in the gradient mode when the mobile phase composition is changing with time as is the pressure drop and solvent heat capacity. Under gradient conditions, a steady state is not reached and retention times of various analytes can be affected. A higher operating pressures, there is a heat backflow through the metal column wall in the opposite direction of solvent flow. The group has done numerical simulations on the flow and heat transport.
A poster paper by a group at Eksigent Technologies, Dublin, California, has proposed a solution to frictional heating by using smaller internal diameter columns in a semi-insulated mounting. To maintain retention time reproducibility and stability, the heat transfer out of the column had to be increased greatly. They developed a theory that predicted the effect of frictional heating at various column diameters and then went on to study frictional heating for various column internal diameters. The experimental results confirmed their theory and they were able to demonstrate stable retention times with expected plate heights for a 0.5-mm i.d. column packed with sub-2-μm particles.
Detection Techniques
By a perusal of the abstracts, I tabulated the detection principles (Table III) that were used in the various presentations at HPLC 2009. Not every abstract indicated the detector that was used so only those that provided this information were counted. The category assignments were based upon the main emphasis of a particular scientific paper as well as separation and detection techniques used. MS clearly dominates the detection category. If one adds up the use of MS in chromatography and electrophoretic techniques, 56% of the papers presented at HPLC 2009 used this detection technique. Compared with last year, there was about a 12% growth in the two MS categories, which were evenly split.

Table III: Types of detection techniques used at HPLC 2009
After MS, as might be expected, UV detection, especially diode array detection, was the second favored detection technique, mostly in application examples. Fluorescence detection and electrochemical detection maintained their level of usage but there was a dramatic falloff in reported applications using evaporative light scattering detection and charged aerosol detection (CAD) this year. Clearly, MS is becoming the detection method of choice in HPLC and CE for the obvious reasons of great selectivity, great sensitivity, and potential for molecular identification.
HPLC 2010 is Next
The next major symposium in this series, the 35rd International Symposium on High Performance Liquid Phase Separations and Related Techniques (HPLC 2010), moves back to the United States and will be held in Boston, Massachusetts on June 19–24, 2010. The chairman of this upcoming event will be Dr. Steven Cohen of the Waters Corporation, Milford, Massachusetts. For more information consult the official website http://www.hplc2010.com/.
Acknowledgement
I would like to acknowledge the contributions my Agilent colleagues Maureen Joseph and John Henderson of Wilmington, Delaware, who supplied detailed notes on some of the oral presentations. I also would like to give a special thanks to Prof. Carol Collins of State University of Campinas, Sao Paulo, Brazil for her excellent typewritten summary of many of the columns' talks that I was unable to attend. Also, thanks to Wolfgang Grysa, the official photographer, for supplying the photos of the award winners.
Ronald E. Majors "Column Watch" Editor Ronald E. Majors is business development manager, Consumables and Accessories Business Unit, Agilent Technologies, Wilmington, Delaware, and is a member of LCGC's editorial advisory board. Direct correspondence about this column to "Sample Prep Perspectives," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgcmag.com.
References
(1) R.E. Majors, LCGC 26(8), 676–691(2008).
(2) R.E. Majors, LCGC 26(9), 898–912 (2008).
(3) R.E. Majors, LCGC 25(9), 920–942 (2007).
(4) R.E. Majors, LCGC 25(10) 1000–1012 (2007).
(5) R.E. Majors, LCGC 24(9), 970–985 (2006).
(6) R E. Majors, LCGC 18(11), 1122–1134 (2000).
(7) R.E. Majors, LCGC 19(10), 1034–1048 (2001).
(8) R.E. Majors, LCGC 20(9), 830–841 (2002).
(9) R.E. Majors, LCGC 21(9), 872–887 (2003).
(10) R.E. Majors, LCGC 22(9), 870–882 (2004).
(11) R.E. Majors, LCGC 23(9), 989–1005 (2005).
(12) R.E. Majors, LCGC 26(S4), 10–17 (2008).
(13) F.M. Rabel, A. Caputo, and E.T. Butts, J.Chromatogr. 126, 731–740 (1976).
Addendum
In June, 2009, I wrote an article for "Column Watch" entitled "The Continuing Acetonitrile Shortage: How to Combat It or Live with It". In this article, I outlined some possible solutions to the worldwide shortage of acetonitrile (ACN). Since the original article, there has been some relief in the supply of this well-used solvent, depending on location. For example, I just returned from South Africa where the shortage is still evident. One observation, though, is that the price has not returned to summer, 2008 levels.
After the article was published, I received some excellent inputs on other alternatives from some readers and I would like to share them with you. Columnist John Dolan of LC Resources brought up the dipolar nature of ACN that wasn't mentioned in the original article. Although the solvent strength of ACN may be matched by other solvent combinations, if solvent chemical properties are important, it may be difficult to find a replacement. John felt that changing other chemical aspects of the system (i.e. temperature, column, pH) might be a better bet.
Reader George E. Tarr of PproSeeQ felt that the substitution of ACN by EtOH could be an alternative solvent that is a better match in solvent strength than MeOH, as was depicted in Figure 5 in my article. Although I mentioned the simple distillation of acetonitrile:water that gives an 86:14 ACN:water binary azeotrope, quite suitable for "B" solvent in many binary gradient systems, Dr. Tarr added that another possibility could be the ternary azeotrope of acetonitrile:ethanol:water. This azeotrope distills around 3.6 degrees Centigrade lower that the binary acetonitrile:water azeotrope and yields a 44:55:1 ternary mixture that would not only spare more than half of the ACN but allow solvent recovery by simple distillation. In his laboratory, using a small pore C18 column, retention times were slightly longer than those with ACN alone, resolution was about the same, the pressure-%B curve was intermediate between ACN and EtOH alone, and the UV cutoff was below 210 nm. A shift in selectivity would be expected but could be good or bad, depending upon the specific case.
Jon Wong of the U.S. Food and Drug Administration (FDA), College Park, MD has been using a mixture of acetone/ethyl acetate/cyclohexane (2:1:1) as a replacement for ACN in the QuEChERS, a growing technique for the extraction of pesticides in fruits and vegetables. His laboratory has found that the recoveries are similar to those of ACN, although he noticed that there were some interferences with this mixture with early eluting compounds. He is currently validating the procedure using both GC- and HPLC-MS/MS. Jon's colleagues at the FDA in Atlanta, Georgia, Frank Schenck and Narong Chamkasem, have also reported the successful use of the acetone/ethyl acetate/cyclohexane (2:1:1) for QuEChERS extraction also as a replacement for ACN.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
Altering Capillary Gas Chromatography Systems Using Silicon Pneumatic Microvalves
May 5th 2025Many multi-column gas chromatography systems use two-position multi-port switching valves, which can suffer from delays in valve switching. Shimadzu researchers aimed to create a new sampling and switching module for these systems.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














