High-Throughput Tools and Approaches for Development of Process Chromatography Steps
LCGC North America
An overview of high-throughput process development (HTPD) tools and approaches, as well as a case study
Quality by Design (QbD) implementation requires more-extensive experimentation to be able to establish the design space for the process and the product. With the biotechnology companies under pressure to contain cost of manufacturing, the use of high-throughput tools has emerged as a necessary enabler of QbD.
The biotechnology industry is gradually adopting the elevated regulatory expectations that have been laid out via initiatives such as the Quality by Design (QbD) and Process Analytical Technology (PAT) in the past decade (1–4). This requires enhanced process and product understanding that comes from a more extensive experimentation than that which traditionally has been performed. Parallel to these rising expectations, the biopharmaceutical industry has been under increasing scrutiny and pressure to contain the cost of healthcare, especially in developed economies. This has renewed the focus of process development efforts toward cost reduction and efficiency improvement. There is a growing need for technologies that can provide us with the required data (information) within the constraints on resources, time, and cost that exist at biopharmaceutical companies today. High-throughput process development tools and approaches fit the bill and appear to offer a solution to this challenge.
Advances in upstream process development leading to increases in fermentation and cell culture titers have shifted the bottleneck of process development to downstream processing of biologicals. Chromatography is perhaps the most significant unit operation in downstream processing of biomolecules, often the primary step for purification. Simplicity of the operation, easy scaleup, cost effectiveness, and robust performance are some of the advantages associated with chromatographic purification. However, optimization of chromatography steps is often a resource- and time-intensive task, and it involves identification of the main effects of the various process parameters. Furthermore, interactions of the process parameters have significant impact on product quality and process consistency. A high-throughput process development (HTPD) platform involving use of miniaturized instrumentation coupled with automated liquid handling devices provides a parallel and efficient way for process development (5).
This column installment aims to provide an overview of the various HTPD tools and approaches that are commercially available today. We hope that this will be of interest to the practitioners in academia and industry that are engaged in process development of biotechnology products. We also present a case study to illustrate practical applications of these approaches.
The Role of Chromatography in Bioprocessing
Purification methods for therapeutic biotechnology products are focused primarily on separation of the various entities that are present at the microbial fermentation or mammalian cell culture stage of process development. These include product modifications such as PEGylation, glycosylation, and acylation; undesirable host cell–related modifications such as truncation, acylation, and methylation; and undesirable process modifications such as aggregation, oxidation, and deamidation. Furthermore, there are numerous host cell–related (host-cell proteins, DNA, viruses, and so forth) and process-related (antifoaming agents, extractables, leachables, and so forth) impurities that may be present in the final product. Each of these species can impact the safety or efficacy of the biotech product, and hence it is critical for the product to have sufficiently high purity and for the process to consistently meet the quality expectations (6).
Process chromatography has remained the mainstay for purifying and separating the product from the above-mentioned variants and impurities. The mechanistic basis of separation can be differential ionic or hydrophobic interactions or a combination of both. Size-based separations can also be performed. Table I lists the different types of chromatographic separations based on the mode of action, along with some of the key process parameters that impact their performances. A large variety of modes of action is available in the commercial market, and for each mode of action, there are many resins available from each of the major vendors of chromatography media. This variety provides practitioners with enormous flexibility in choosing the optimal media for a given application, and it is a key reason why chromatographic steps have established themselves as the workhorses of downstream processing of biological molecules.

Table I: Modes of action and significant process parameters that affect different types of chromatography
Chromatography is performed most commonly in a column format, where the column is packed with spherical beads (resins). More recently, membrane chromatography has emerged as an alternative that has been shown to work for a subset of the applications. The latter involves use of synthetic microporous or macroporous membranes as chromatography media and offers low pressure drop along with reduced radial and axial dispersion, in comparison to traditional chromatography (7).
A Need for High-Throughput Process Development
Process chromatography optimization mainly involves knowledge-based, algorithm-based, or high-throughput process development (8). However, as a rule of thumb, most industrial process development involves integration of all three aspects of process development, leading to a hybrid protocol for process development and optimization. Process parameters and the level selection of HTPD experiments mainly depend on prior knowledge or experience, as well as on appropriate theory-based assumptions. A high-throughput process development platform for chromatography helps in the rapid optimization of process parameters by integration of miniaturization, parallelization, and automation (5). Miniaturization involves the use of microelectronics to enable greater operational flexibility, reduce the required reagent volumes, and increase productivity of experimentation. Automation in fluid handling and analytical detection results in higher productivity, accuracy, and laboratory safety. Parallelization involves performing several tasks at the same time and results in a massive increase in productivity with respect to experimentation.
Irrespective of the format and the mode of action, optimization of the chromatographic step is the critical task, and it involves the simultaneous optimization of the number of process parameters affecting the separation of the molecule (Table I). A typical chromatography step can have 5–20 input process parameters, 5–10 output parameters, and may use 5–20 raw materials. As such, risk analysis is often performed based on prior experience and scientific knowledge to reduce the number of variables to be examined to 4–10. Still, a full factorial study for six parameters requires as many as 64 experiments, and performing these one at a time using traditional approaches is a resource- and time-intensive task.
Commercial, Miniaturized Platforms Currently Available for Enabling HTPD in Chromatography
Miniaturized platforms for HTPD in chromatography are classified into three different types, depending upon the geometry and the fluid flow mechanisms involved (9): a 96-well format, miniaturized chromatography columns, and micropipette tip-based columns.
96-Deep-Well Format: Two 96-deep-well plates are available for HTPD: PreDictor plates from GE Healthcare (Piscataway, New Jersey) and AcroPrep multiwell plates from Pall Corporation (Port Washington, New York).
PreDictor plates provide a platform for HTPD by allowing for the parallel screening of chromatographic conditions, either in a manual or automated workflow. These are disposable plates, and they are available with a variety of volumes of filled resin in the wells, starting at 2 µL. The setup enables screening of a large experimental space to identify the subspace that is optimal for the application under consideration (10). PreDictor plates, along with the company's Assist software, offer a simultaneous experimentation and data analysis tool that can enable QbD-based process development.
The AcroPrep multiwell plate format is available in two filled resin volumes of 350 µL and 1 mL with a pore size of 0.45 µm. The usual material of construction for multiwell plates is a hydrophilic polymer, GHP (hydrophilic polypropylene), or Supor (hydrophilic polyethersulfone), leading to minimal adsorption of protein molecules and, thus, preventing nonspecific interactions such as hydrophobic ones. This plate also provides a high-throughput tool for optimization of ion-exchange membrane chromatography. The plates are available with the company's Mustang Q and S high-throughput membrane chromatography platforms for anion- and cation-exchange membrane chromatography optimization, respectively (11).
Miniaturized chromatography columns: The MediaScout MiniColumn platform from ATOLL-bio USA (Gloucester, Massachusetts) provides a miniaturized chromatography column alternative for performing HTPD. This platform consists of an array of small columns installed on a base plate, which can be customized for the particular type of resin, depending on the user's requirements. Three versions are available, namely, the CentriColumn, PipetColumn, and RoboColumn, which mainly differ in the manner in which liquid is dispensed in the column (12).
Micropipette tip-based columns: PhyNexus Inc. (San Jose, California) has developed unique columns to provide an HTPD solution. The PhyTip columns are available for various modes of chromatography, including gel filtration chromatography (GFC). Micropipette tip-based columns offer a solution for effective sample preparation for various analytical techniques (13).
Applications of HTPD for Chromatography Optimization
Downstream process development for a biological molecule mainly involves a combination of two to five different chromatographic steps arranged in a specific sequence. Each step is designed to serve a unique purpose, such as to remove a particular impurity or a contaminant. HTPD helps in the screening of the different modes of chromatography for a given application, the screening of different resins for a given mode of chromatography, and finally, optimization of the chromatography step in a timely, efficient manner. In the following sections, we review the literature and provide guidance for performing HTPD for each of the commonly used modes of chromatography.
Ion-Exchange Chromatography: Wiendahl and colleagues (14) have used the MediaScout RoboColumn integrated with a Tecan EVO freedom liquid handling station (Tecan Group Ltd., Männedorf, Switzerland) for dynamic chromatographic operations, such as breakthrough-curve determinations and elution experiments. The efficiency of the HTPD platform for protein separation was studied using three model systems: separation of BSA and lipolase, separation of human growth hormone and its precursor, and separation of insulin analog precursor and a related contaminant. Good agreement was observed among the results obtained for the elution experiments done using the HTPD platform and the results obtained using 1-mL prepacked columns with an äKTAexplorer liquid chromatography system (GE Healthcare) (14). Stein and Kiesewetter (15) have evaluated the HTPD platform for identifying the alternatives to protein A chromatography, a critical step for purification of monoclonal antibody (MAb) products. The influence of pH on binding of monoclonal antibodies and removal of host-cell proteins (HCP) was evaluated using two strong-cation-exchange resins with a special geometry (15). Their results again demonstrated the usefulness and effectiveness of using HTPD platforms.
Sensitivity of the analytical technique is a critical factor affecting selection of an HTPD method over conventional chromatography process development protocols. An HTPD platform was utilized efficiently with surface-enhanced laser desorption–ionization time-of-flight (SELDI-TOF) mass spectrometry (MS) as the analytical technique. This approach resulted in a significant reduction in analysis times and improvements in resolution (16). Susanto and colleagues (17) have proposed a closed-loop optimization strategy for high-throughput experimentation that combines an automated liquid handling system with a genetic algorithm. This optimization protocol highlights the importance of using demonstrated knowledge and computational algorithms in the appropriate experimental design selection and effective process development. Dynamic binding capacity determination under the various process conditions is one of the critical tasks in process development. Bergander and colleagues (18) have determined the dynamic binding capacities of a human polyclonal IgG on MabSelect SuRe (GE Healthcare) and amyloglucosidase on Capto DEAE resins (GE Healthcare) using an HTPD platform.
Hydrophobic Interaction Chromatography: An HTPD platform has been used for screening of different hydrophobic interaction chromatography (HIC) resins for separating a monoclonal antibody from aggregated species. The type of resin matrix, hydrophobicity of the ligand, density of the ligands, chemistry of the backbone, and mobile phase variables such as pH, salt type, and salt concentration were some of the process parameters that were examined for optimization of the HIC step (19).
Affinity Chromatography: An HTPD platform has been used for development of a protein A chromatography step to capture a monoclonal antibody product, which was developed to enable characterization of IgG2 (20).
Limitations of HTPD for Chromatography
Although the efficiency offered by use of HTPD systems and approaches is promising, the currently available technologies have yet to mature and offer improved robustness. Existing tools continue to present significant limitations. Manual processing of buffers and protein samples may lead to excessive noise during the experimentation and result in errors in data analysis. This fact highlights the need for automation during equilibration, protein loading, and elution phases of process development. Also, an HTPD system is not yet a suitable alternative for reversed-phase chromatography and size-exclusion chromatography (SEC) because of the need for volatile mobile phases in reversed-phase chromatography and large theoretical plates in SEC (21). Finally, an estimation of protein purity is a significant hurdle in HTPD, thereby limiting the overall throughput of the process (21).
Development of a Typical HTPD Protocol
Figure 1 illustrates the various steps that are involved in creating an HTPD protocol. In the following sections we discuss them in more detail.
Phase I: Pre-experimental Planning: Pre-experimental planning consists of tasks such as identifying a suitable resin matrix and selecting the process parameters that need to be examined and the appropriate levels that need to be evaluated. After these inputs are known, the appropriate design of experiments (DOE) can be chosen and the experimental plan can be created manually or by using commercially available DOE software.
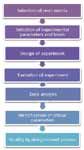
Figure 1: Steps involved in HTPD for chromatography optimization.
Phase II: Experimentation: This phase starts with a sample preparation protocol that involves concentration and buffer exchange of the protein of interest into the various combinations of buffer, as required by the DOE protocol. Experiments are performed according to the protocol and include equilibration, protein loading, and elution cycles. As mentioned above, the availability of analytical instrumentation having the necessary sensitivity and ability to analyze HTPD formats is essential for achieving satisfactory mass balance and accurately estimating recovery and product quality.

Table II: Operating conditions for the design of experiments study using HTPD
Phase III: Data Analysis: Automation and parallelization of experiments in an HTPD platform make data analysis a complex task. Statistical data analysis tools help with interpreting the data and defining the optimum operating conditions. Care should be taken to recognize appropriately the various errors that can come from working with such small volumes.
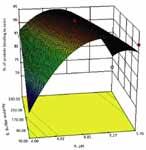
Figure 2: Interactions between pH and amount of protein added on the binding of a biosimilar protein to resin.
Case Study: Optimization of Binding Conditions for a Recombinant Protein on a Multimodal Resin
This case study involved a screening of binding conditions for a chromatography step involving the use of a multimodal resin for purification of a low molecular weight, biosimilar product expressed in E. coli. Such a study typically involves the selection of a buffer system, optimization of the binding pH, investigation of the ionic strength of the buffer system, protein loading per unit volume of resin, and concentration of the various additives that may enhance product binding. Knowledge-based, selective elimination of factors resulted in identification of three process parameters for further investigation, namely binding buffer pH, binding buffer ionic strength, and the protein loading per unit volume of resin. Table II lists the selected ranges for each of the process parameters that were identified for investigation. Figures 2 and 3 illustrate the effect of these parameters on the binding of the product to the resin. The benefits of the DOE are clear, as both the main effects and the interactions between the different process parameters can be observed. Figure 2 shows that the effect of protein loading is minimal, an increase in buffer molarity results in a decrease in binding, and optimal binding is observed with respect to pH. Although the figure shows that there is no interaction between pH and protein loading, there is a significant amount of interaction between pH and buffer molarity. Table III presents a comparison of some of the efficiency metrics for performing this study, now using a traditional vs. an HTPD protocol. The table shows that there is an order of magnitude difference in the time required to perform the experiments by the two approaches. An even greater difference (100-fold) is seen with respect to the amount of product used for the study.
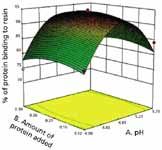
Figure 3: Interaction between pH and buffer molarity on the binding of a biosimilar protein to resin.
Summary
We hope that this column installment familiarizes readers with the various HTPD approaches that are commercially available for the development of chromatography steps. We also hope that we have been able to demonstrate the advantages of using HTPD with respect to savings in time and feedstock materials. While these technologies are still at a stage of early adoption, particularly in the area of bioprocessing, we are confident that they will undergo major development over the next decade. HTPD will change dramatically how we perform the various activities required for product and process development and commercialization.
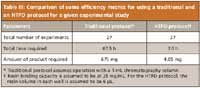
Table III: Comparison of some efficiency metrics for using a traditional and an HTPD protocol for a given experimental study
Rahul Bhambure is a graduate student in the Department of Chemical Engineering at the Indian Institute of Technology, Hauz Khas, New Delhi, India.

Rahul Bhambure
Ira S. Krull "Biotechnology Today" Co-Editor Ira S. Krull is an Associate Professor of chemistry at Northeastern University, Boston, Massachusetts, and a member of LCGC's editorial advisory board.

Ira S. Krull
Anurag S. Rathore is a biotech CMC consultant and an associate professor with the Department of Chemical Engineering, Indian Institute of Delhi, India. He is also a member of BioPharm International's editorial advisory board.

Anurag S. Rathore
References
(1) A.S. Rathore and H. Winkle, Nat. Biotechnol. 27, 26–34 (2009).
(2) A.S. Rathore, Trends Biotechnol. 27, 546–553 (2009).
(3) E.K. Read et al., Biotechnol. Bioeng. 105, 276–284 (2010).
(4) E.K. Read et al., Biotechnol. Bioeng. 105, 285–295 (2010).
(5) R. Bhambure et al., Trends Biotechnol., (2011). doi:10.1016/j.tibtech.2010.12.001.
(6) A.S. Rathore and I.S. Krull, LCGC North America 28(8), 598–608 (2010).
(7) U. Gottschalk, Biotechnol. Prog. 24, 496–503 (2008).
(8) B.K. Nfor et al., Trends Biotechnol. 27(12), 673–679 (2009).
(9) S. Chhatre and Nigel J. Titchener-Hooker, J. Chem. Technol. Biotechnol. 84, 927–940 (2009).
(10) http://www.gelifesciences.com/aptrix/upp01077.nsf/content/bioprocess~columns~predictor#
(11) http://labfilters.pall.com/catalog/laboratory_19999.asp
(12) http://www.atoll-bio.com/
(13) http://www.phynexus.com/products/
(14) M. Wiendahl et al., Chem. Eng. Technol. 31(6), 893–903 (2008).
(15) A. Stein, and A. Kiesewetter, J. Chromatogr. B. 848(1), 151–158 (2007).
(16) P.S. Wierling et al., Biotechnol. Bioeng. 98(2), 440–450 (2007).
(17) A. Susanto et al., Chem.Eng.Technol. 32, 140–154 (2009).
(18) T. Bergander et al., Biotechnol. Prog. 24, 632–639 (2008).
(19) J.F Kramarczyk et al., Biotechnol Bioeng. 100(4), 702–720 (2008).
(20) M.J. Bailey et al., J. Chromatogr. B. 826, 177–187 (2005).
(21) K. Rege and M. Heng, Nat. Protoc. 5(3), 408–417 (2010).

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











