HILIC–MS Sensitivity without Silica
LCGC North America
What challenges occur when using hydrophilic interaction liquid chromatography (HILIC), and what options are available for analysis?
Recent developments in the manufacturing of high performance liquid chromatography stationary phases have created polar stationary phases that have the ability to retain certain polar species. However, these stationary phases still require highly aqueous mobile phases, which cause reduced mass spectrometry (MS) sensitivity. Hydrophilic interaction liquid chromatography (HILIC), which uses a polar stationary phase, has proved to be an effective approach in improving MS sensitivity, while presenting certain drawbacks. Porous graphitic carbon can retain very polar and ionic species as well as hydrophobic analytes. Here we focus on the challenges faced with the retention of "problematic" polar species and consider the options available for their analysis.
Small polar analytes have proved difficult to analyze due to lack of retention on conventional reversed-phase columns. In conventional reversed-phase systems, analyte retention increases as its hydrophobicity increases. Typical reversed-phase materials are hydrophobic and have been developed for the retention and separation of hydrophobic compounds. On these phases, polar–ionizable compounds are eluted at or near the dead volume of the column, making effective analysis of these compounds challenging. Approaches to induce retention of polar compounds include solute derivatization, in which a chemical reaction is used to convert the polar species to a more hydrophobic compound, and ion-pairing, which uses a reagent of the opposite charge in the mobile phase to create a neutral species. Derivatization is time consuming, and ion pairing has disadvantages when mass spectrometry (MS) detection is used, namely, ionization suppression and contamination of the ion source with involatile reagents. Recent developments in the manufacturing of stationary phases have created phases with some polar character, which have the ability to retain certain polar species. However, retention of very polar species on these columns still may require the use of highly aqueous mobile phases, which leads to reduced MS sensitivity.

Hydrophilic interaction liquid chromatography (HILIC), which uses a polar stationary phase, has become an increasingly popular alternative approach to effectively retain and separate small polar species. Stationary phases that have been used for HILIC include unmodified silica and silica or polymeric supports modified with functional groups such as hydride, aminopropyl, cyanopropyl, diol, amides, sulfonic acid, poly(succinimide), and sulfoalkylbetaine zwitterionic (1). A disadvantage of HILIC is that it can retain only polar species, while hydrophobic species are eluted at or just after the column void volume. There are some stationary phases that can retain both hydrophilic and hydrophobic species. Porous graphitic carbon (PGC) offers a further alternative, because it can retain hydrophobic analytes as well as very polar and ionic species through a specific retention mechanism. It retains hydrophobic species through dispersive interactions and polar species through dipole–dipole interactions in both reversed phase–type mobile phases or HILIC-type mobile phases.
HILIC Considerations
Mechanism: Alpert (2) first proposed the acronym HILIC to describe a chromatographic technique in which the analytes interact with a hydrophilic stationary phase and are eluted using a relatively hydrophobic mobile phase with water as the strong eluent. Water is present at levels of 5–40%, forming a polar layer semi-immobilized onto the surface of the stationary phase into which the polar analytes partition (1). The evidence for the partition mechanism includes the fact that polar stationary phases, such as silica and modified silicas, when used with an aqueous-organic mobile phase, retain a semi-immobilized layer of water-enriched eluent (3–5). There also is evidence that supports a second mechanism of interaction that contributes to the retention for ionizable compounds in HILIC, ion exchange. McCally (6) has demonstrated significant changes in the selectivity of ionizable compounds with changes in the buffer and pH of the aqueous component of the mobile phase. The elution order in HILIC is generally the opposite of that in reversed-phase liquid chromatography (LC) and similar to normal-phase chromatography, in which the more polar the analyte, the greater the retention. Alpert (2) has reported an almost complete reversal of the elution order of amino acids in HILIC as compared to reversed-phase chromatography.
In HILIC mode, retention of polar analytes increases as the quantity of nonpolar solvent in the mobile phase (generally acetonitrile) increases. Figure 1 shows this behavior for unmodified silica and polar-bonded silica phases. At low percentage organic in the mobile phase (high water content), there is no or low retention because the hydrophilic compounds prefer to be in the mobile phase. As the percentage of acetonitrile increases, the analyte partitions into the water-enriched layer of stagnant eluent on the hydrophilic stationary phase, and retention is observed.

Figure 1: HILIC retention behavior on (a) unmodified and (b) polar-bonded silica phases. Shown are plots of the percentage of acetonitrile in the mobile phase versus the retention factor. Column dimensions: 150 mm à 4.6 mm, 5 µm; mobile phase: 10 mM ammonium acetate (pH 5.0)âacetonitrile; flow rate: 0.6 mL/min; detection: UV absorbance at 254 nm; temperature: 30 °C.
Improved MS Sensitivity: HILIC, therefore, offers the advantage of being able to retain very hydrophilic compounds, such as urea and lactulose (7), that are not retained in conventional reversed-phase chromatography. Additionally, the mobile phases have high organic content, enabling improved sensitivity with MS detection by virtue of the fact that highly organic mobile phases ensure efficient desolvation. An additional advantage of using highly organic mobile phases is a lower pressure drop across the column, which enables the use of higher flow rates to increase analysis throughput.
The improvement in the sensitivity of HILIC–MS analysis is illustrated using nicotine and cotinine in Figure 2. The mobile phases in reversed-phase chromatography and HILIC have been optimized to obtain retention and resolution of the two species. In the reversed-phase analysis, only 2% organic modifier (the strong eluent) is used in the mobile phase because a higher organic content would result in elution of the two analytes at the column void volume. In HILIC analysis, the mobile phase contains 10% of the strong eluent (100 mM buffer) and 90% of acetonitrile (the weak eluent). The signal-to-noise ratio (S/N) for nicotine is 15 times higher in HILIC than in reversed-phase LC; the S/N for cotinine is five times higher in HILIC than in reversed-phase LC.

Figure 2: Comparison of the MS sensitivity obtained for the analysis of nicotine and cotinine in (a) reversed-phase LC and (b) HILIC modes. (a) Column: 150 mm à 2.1 mm, 5-µm Hypersil GOLD, mobile phase: 98:2 waterâacetonitrile plus 0.1% formic acid; detection: +ESI (spray conditions adjusted for higher aqueous content of the mobile phase); injection: 1 ng on column. (b) Column: 150 mm à 2.1 mm, 5-µm polar-bonded silica; mobile phase: 10:90 50 mM ammonium formate (pH 3.5)âacetonitrile; detection: +ESI; injection: 1 ng on column.
Challenges: One of the challenges of HILIC is that it cannot separate mixtures of hydrophilic and hydrophobic compounds. It is effectively the opposite to reversed-phase LC, because only hydrophilic compounds can be retained and separated; hydrophobic compounds are not retained. Chromatographers are faced with a choice of which analytes to retain: hydrophobic by reversed phase or hydrophilic by HILIC. Furthermore, we have recently observed additional problems with the use of HILIC regarding method robustness (8). These include retention time reproducibility and column equilibration and storage.
Column equilibration can prove problematic, with the system requiring many column volumes to reach a steady state. Figure 3 shows that the time taken for the unmodified silica column to equilibrate is prohibitively long. Approximately 400 column volumes of solvent (equating in this experiment to a period of 10 h) were required to condition the column before reproducible retention time results could be obtained for analysis. With respect to stability following conditioning, deterioration in retention time was not observed for this media with exposure to as much as 800 column volumes of mobile phase.
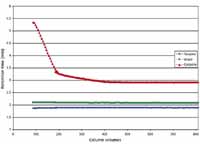
Figure 3: Column equilibration on unmodified silica in HILIC mode: retention time variation for toluene, uracil, and cytosine as a function of the number of column volumes of mobile phase through the column. Column: 150 mm à 4.6 mm, 5-µm unmodified silica; mobile phase: 50:50 10 mM ammonium acetate (pH 5.0)âacetonitrile; flow rate: 1.0 mL/min; detection: UV absorbance at 254 nm; temperature: 25 °C. Analytes: toluene, uracil, and cytosine.
Associated with column equilibration, retention time drift was observed for both unmodified silica materials and polar-bonded stationary phases. Our studies have investigated column stability using a set of three polar compounds as test probes. For the last-eluted compound acyclovir, a variation was observed in retention times from 5.56 to 6.09 min during the course of 180 injections. This equates to approximately a 10% change. The column was left flushing with the mobile phase overnight and the following morning injection of the same test mix revealed a further shift in the retention of acyclovir to 6.35 min. This common issue can be alleviated by increasing the concentration of buffer; however, there are limitations of buffer solubility in the highly organic mobile phase, and MS sensitivity can be reduced. During the process of studying the column stability, we also observed that very small variations in the mobile phase composition can affect the retention time precision of the method.
Our studies also highlighted a change in capacity factor after storing the unmodified silica column in the HILIC mobile phase, without the buffer, for a period of three weeks. A change of 24% in the capacity factor of cytosine was observed, in addition to increased peak asymmetry for this compound.
Retention and Selectivity: Recent studies also have shown different retention characteristics for traditionally difficult, polar model compounds on four different polar stationary phases (amide, amino, silica, and sulfobetaine) in HILIC separation (9). The silica phase was the least retentive of the four phases and exhibited unique selectivity for the nucleosides and bases, possibly because of secondary interactions between the solutes and surface silanol groups. The data showed that functional groups on the stationary phases might have certain degrees of influence on selectivity, possibly through secondary interactions with the model compounds. The study on the effect of buffer salt concentration also found an across-the-board increase in retention with the salt concentration in the mobile phase, except for the acids on the amino phase. This was in sharp contrast to reversed-phase chromatography, where the retention of uncharged compounds generally was not affected significantly by the buffer salt concentration.
Perspectives on Porous Graphitic Carbon
An alternative solution for the retention of hydrophilic species is the use of porous graphitic carbon. PGC can retain polar species in both typical reversed-phase and HILIC mobile phases. This retention of hydrophilic compounds in organic-rich mobile phases, such as the ones used in HILIC, can be described as an aqueous normal-phase mechanism (10).
Mechanisms: PGC holds unique properties as a stationary phase in high performance liquid chromatography (HPLC), providing exceptional retention and separation of highly polar compounds. PGC also behaves in a similar manner to a strongly retentive alkyl-bonded phase for non-polar analytes. Its flat, highly crystalline surface leads to retention mechanisms that are different from those observed on silica-based bonded phases. The retention mechanisms involved are a combination of dispersive interactions between the analyte–mobile phase and analyte–graphitic surface, by which retention increases as the hydrophobicity of the molecule increases, together with charge-induced interactions of polar analytes with the polarizable surface of the graphite (11).
These two mechanisms of interaction (dispersive and polar retention) explain the capacity of PGC to separate mixtures of compounds with a wide range of polarity, such as water and fat soluble vitamins, in a single chromatographic run using generic mobile phase conditions (12). Uracil also provides evidence of PGC's unique properties, as it is traditionally used as a t0 marker for alkyl chain silica phases because it exhibits no retention on these phases. On PGC, uracil, which has a log P of –1.0, is well retained (capacity factor of approximately 3) using a simple mobile phase gradient of water and acetonitrile with 0.1% formic acid (12).
Physical Properties: PGC's physical properties are similar to other HPLC supports, meeting all conventional operating
criteria. It is manufactured using a silica template to create spherical, porous particles with 250-å pores. This tight pore-size distribution ensures good mass transfer of a large number of analyte shapes and sizes.
The surface area of PGC is relatively small (120 m2 /g) in comparison with most silica-based stationary phases, which tend to have surface areas closer to 300 m2 /g. However, the retention mechanism provided by the PGC surface works quite differently from that of silica and still provides strong retention. Because it is 100% carbon, PGC is chemically very robust with complete pH stability, remaining stable under extreme conditions of buffer concentration and temperature. It can be used for normal-phase and reversed-phase LC and provides a long column lifetime. Furthermore, there are no silanols that might otherwise produce secondary interactions with analytes.
Polar Retention Effect on Graphite: Retention on PGC depends on the polarity of the molecule, which is a key advantage of this stationary phase.
The strength of analyte interactions with PGC is largely dependent on the molecular area in contact with the graphite surface, and also on the type and positioning of the functional groups in relation to the graphite surface at the points of contact. The polarizability of the surface of graphite is considered the key to understanding the mechanism by which polar compounds are retained (13). Values have been calculated pertaining to the polar retention effect on graphite by comparing retention behavior of test polar solutes with that of equivalent hydrocarbons (14,15). Polar molecules have a permanent dipole and thus can induce a dipole on the polarizable surface of the graphite as they approach it, which increases the attraction between the analyte and the graphite surface. This unusually high retention of polar analytes can be described as the polar retention effect on graphite (PREG).
It is this property that makes PGC columns particularly useful for the separation of highly polar compounds that otherwise would be difficult to retain on typical C18 packings. PGC columns can provide a workable alternative to ion-exchange chromatography for compounds that are ionizable and a viable choice for the retention of highly polar compounds, including carbohydrates and compounds containing numerous hydroxyl, carbonyl, and amino functional groups.
Coquart and Hennion (16–18) have carried out in-depth studies into the retention of polar hydroxy-substituted benzenes. Their work demonstrated how differently PGC columns retain polar compounds compared to silica-based C18 and poly(styrene–divinylbenzene) (PS–DVB), a polymer-based reversed-phase material. The polar retention effect on PGC is displayed in Figure 4 (17), where the retention (log k) was plotted against the percentage of methanol in the mobile phase (water–methanol) for a series of polar compounds (mono-, di-, and trisubstituted benzenes). Log P is a physical parameter used to describe a compound's hydrophobic properties. The lower the log P value, the less hydrophobic the compound (that is, the more polar), and thus, the less retention is obtained in reversed-phase with conventional phases. On the reversed-phase resin, the most polar compound (phloroglucinol, with a log P of 0.16) is eluted first (as expected), so the elution order is phloroglucinol–resorcinol–phenol. Phloroglucinol needs less than 20% organic to be retained. Conversely, the PREG means that phloroglucinol is the most retained analyte on the PGC column.

Figure 4: Polar retention effect on graphite (PREG). Comparison of the capacity factor and elution order of phenol, resorcinol, and phloroglucinol on (a) PS-DVB and (b) PGC (Hypercarb). Log P: phenol, 1.46; resorcinol, 0.8; phloroglucinol, 0.16. (Courtesy of V. Cocquart and M.-C. Hennion, 1992.)
Retention as a Function of % Organic: PGC, which has a hydrophobic surface, displays both typical reversed- and normal-phase mode retention characteristics (Figure 5). Analyte retention first decreases as the percentage of organic increases (typical reversed-phase behavior) and then increases as the concentration of acetonitrile becomes greater. The latter effect mimics normal-phase behavior because the retention increases when the mobile phase becomes more nonpolar. HILIC only provides the latter and not the former modes. It is this dual behavior that enables PGC to be used for the separation of polar analytes under either reversed-phase or aqueous normal-phase conditions.

Figure 5: Effect of the percentage of acetonitrile in the mobile phase on the retention capacity on PGC. Column dimensions: 100 mm à 2.1 mm, 3 µm; mobile phase: 10 mM ammonium acetate (pH 4.7)âacetonitrile; flow rate: 0.2 mL/min; column temperature: 40 °C; detection: ESI-MS.
Sensitivity Comparison with HILIC: For the analysis of cyclic monophosphates, unmodified silica in HILIC mode was found to yield very slightly greater sensitivity in LC–MS compared to PGC; however, the resultant peak shape was inferior (Figure 6). Resolution of analytes using unmodified silica in HILIC was only achievable at 90% acetonitrile; consequently, a direct comparison with the 30:70 aqueous–buffer mobile phase used with PGC was not possible. The total analysis time achieved for unmodified silica was more than four times greater than required by PGC.
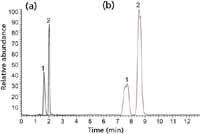
Figure 6: Comparison of the MS sensitivity of the analysis of cyclic monophosphates in (a) reversed-phase LC on a PGC column and (b) HILIC with an unmodified silica column. Column (a): 100 mm à 2.1 mm, 5-µm Hypercarb; column (b): 150 mm à 2.1 mm, 5-µm unmodified silica; mobile phase: 20 mM ammonium acetateâacetonitrile (30:70 for PGC, 10:90 for unmodified silica); flow rate: 200 µL/min; detection: +ESI (normalized scale); temperature: 30 °C. Peaks: 1 = 3',5'-cGMP, 2 = 3',5'-cAMP.
Robustness Compared with HILIC: In marked contrast to the previous findings with HILIC on an unmodified silica column (Figure 3), the PGC column equilibrated rapidly. Consistent retention times were obtained within 25–30 column volumes of mobile phase (equating to 25–30 min). With respect to stability, deterioration in retention time was not observed for either stationary phase after exposure to as much as 800 column volumes of mobile phase.
Retention Time and Selectivity Comparison: A comparison of capacity factors was undertaken in which the retention of the polar molecule allantoin in HILIC was compared with its retention in reversed-phase LC on PGC. A capacity factor of less than 1 was obtained in HILIC with a very weak mobile phase that contained a minimum amount of aqueous buffer at 5%. In contrast, a capacity factor of 2 was obtained on PGC with a mobile phase containing 5% acetonitrile (7).
PGC in reversed-phase mode and HILIC provide different selectivity for the separation of a range of polar compounds (results obtained from an ongoing study at author's laboratory). For example, the separation of purines and pyrimidines (1 – cytosine, 2 – uracil, 3 – thymine, and 4 – xanthine) on PGC and unmodified silica shows elution order 1,2,3,4 and 3,2,4,1, respectively. Cytosine was the first compound to be eluted from the PGC stationary phase whereas, with the silica, it was the most strongly retained compound. In HILIC mode, cytosine possesses the greatest affinity for the adsorbed aqueous layer, whereas in the PGC separation, cytosine is eluted first because of its polarity and shape. Overall, the unmodified silica phase was superior in terms of peak shape; however, the mobile phase required to achieve separation was not as simple as that required for PGC (a 100 mM ammonium formate [pH 3.0]–acetonitrile gradient versus a water–acetonitrile plus 0.1% formic acid gradient).
PGC can retain hydrophilic compounds in typical reversed-phase conditions of low organic content, and also in aqueous normal-phase conditions with high organic content. Figure 7 shows the separation of four cyclic monophosphates with a reversed-phase gradient from low to high organic (Figure 7a) and an aqueous normal-phase gradient from low to high aqueous (Figure 7b). Interestingly, the same elution order is observed for both modes.

Figure 7: Comparison of (a) reversed-phase and (b) aqueous normal-phase separations of cyclic monophosphates on PGC. Column: 100 mm à 4.6 mm, 5-µm Hypercarb; mobile phase A: 20 mM ammonium acetate (pH 5.5); mobile phase B: acetonitrile; flow rate: 1.24 mL/min; detection: UV absorbance at 254 nm. Gradient (a): 10â60% B in 11.1 min, hold for 2.4 min, then 60â100% B in 3 min. Gradient (b): 90â0% B in 20 min. Peaks: 1 = 3',5'-cCMP, 2 = 3',5'-cUMP, 3 = 3',5'-cGMP, 4 = 3',5'-cAMP.
Conclusions
It is evident from the studies discussed in this article, as well as previous studies, that PGC has proved an effective alternative to HILIC for the retention and separation of polar compounds. It offers certain advantages over HILIC in that it can separate polar and nonpolar compounds in the same chromatographic run and shows better retention time reproducibility and faster column equilibration times than HILIC materials. The MS sensitivity obtained with HILIC and PGC is comparable. Additionally, PGC provides alternative selectivity to HILIC.
Luisa Pereira is a Principal Scientist in Chromatography Consumables and Specialty Products, Thermo Fisher Scientific, Runcorn, Cheshire, United Kingdom. She can be contacted by e-mail at luisa.pereira@thermofisher.com.
References
(1) P. Hemström and K. Irgum, J. Sep. Sci. 29, 1784 (2006).
(2) A.J. Alpert, J. Chromatogr. 499, 17 (1990).
(3) L.A. Verhaar and B.F. Kuster, J. Chromatogr. 234, 57 (1982).
(4) P. Orth and H. Engelhardt, Chromatographia 15, 91 (1982).
(5) L.Z. Nikolov and P.J. Reilly, J. Chromatogr. 325, 287 (1985).
(6) D.V. McCalley, J. Chromatogr. A 1171, 46 (2007)
(7) L. Pereira, work performed at the author's laboratory.
(8) C. Blythe, L. Pereira, D. Milton, and M. Dolci, poster presented at HPLC 2008 Symposium, Baltimore, Maryland, 2008.
(9) Y. Guo and S. Gaiki, J Chromatogr. A 1074, 71–80 (2005).
(10) J. Pesek and M.T. Matyska, LCGC North America 25(5), 480 (2007).
(11) J. Knox and P.Ross, Advances in Chromatography 37, 73 (1997)
(12) Method Development Guide for Hypercarb, TG20394_E 1207M, Thermo Fisher Scientific.
(13) P. Ross, LCGC Europe 13(5), 310 (2000).
(14) P. Ross PhD Thesis, Edinburgh University (Edinburgh, UK, 1999).
(15) J.H. Knox and P. Ross, "Quantification and Correlation of the Energies Associated with Retention of Polar analytes on Porous Graphitic Carbon" poster at HPLC 1999 Granada, Spain, 30 May–4 June 1999.
(16) V.F. Coquart, Ph.D. thesis, University of Paris, (1993).
(17) V.F. Coquart and M.-C. Hennion, J. Chromatogr. 600, 195 (1992).
(18) M.-C Hennion and V.F. Coquart, J. Chromatogr. 642, 211 (1993).

Characterizing Polyamides Using Reversed-Phase Liquid Chromatography
May 5th 2025Polyamides can be difficult to characterize, despite their use in various aspects of everyday life. Vrije Universiteit Amsterdam researchers hoped to address this using a reversed-phase liquid chromatography (RPLC)-based approach.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














