High-Speed Analysis of b-Blockers and Metabolites in Human Plasma by LC–ESI-MS-MS with High-pH Mobile Phase
Special Issues
Beta-blockers are basic compounds that contain a secondary amino group in their structure. The amino substituents are typically an isopropyl group and a larger chain with a hydroxyl group in the beta position from the nitrogen atom (Table I). The simultaneous analysis of ?-blockers in biological samples is meaningful, and is made possible by the similarities in their structure. Gas chromatography (GC)–mass spectrometry (MS) has been the most used technique for their identification and quantification (4–6). However, most ?-blockers are nonvolatile and thus require derivatization via a cumbersome and time-consuming process before GC–MS analysis. In recent years, liquid chromatography (LC) coupled with mass spectrometric detection has evolved as the method of choice for drug analysis in the pharmaceutical, clinical, and forensic toxicology areas (4–8). In contrast to GC–MS, LC–MS-MS generally does not require derivatization and offers superior sensitivity. Moreover, due to the high specificity offered by LC–MS-MS, baseline chromatographic resolution often is not required, allowing for fast analysis in high-throughput environments.
Beta-blockers are basic compounds that contain a secondary amino group in their structure. The amino substituents are typically an isopropyl group and a larger chain with a hydroxyl group in the beta position from the nitrogen atom (Table I). The simultaneous analysis of β-blockers in biological samples is meaningful, and is made possible by the similarities in their structure. Gas chromatography (GC)–mass spectrometry (MS) has been the most used technique for their identification and quantification (4–6). However, most β-blockers are nonvolatile and thus require derivatization via a cumbersome and time-consuming process before GC–MS analysis. In recent years, liquid chromatography (LC) coupled with mass spectrometric detection has evolved as the method of choice for drug analysis in the pharmaceutical, clinical, and forensic toxicology areas (4–8). In contrast to GC–MS, LC–MS-MS generally does not require derivatization and offers superior sensitivity. Moreover, due to the high specificity offered by LC–MS-MS, baseline chromatographic resolution often is not required, allowing for fast analysis in high-throughput environments.
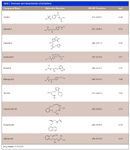
Table I
In this work, a high-speed reversed-phase LC positive ion mode electrospray tandem mass-spectrometric (LC–ESI-MS-MS) method for the quantitative determination of nine β-blockers — acebutolol, alprenolol, atenolol, labetalol, metoprolol, pindolol, propranolol, sotalol, and timolol — in human plasma is presented. Solid-phase extraction (SPE) cartridges are used for sample cleanup and a C18 high performance liquid chromatography (HPLC) column for separation in high-pH mobile phase. The novelty of our approach resides in the choice of mobile phase. Most often, basic compounds are analyzed by LC–MS-MS in acidic mobile phase in which the analytes are positively charged. Unfortunately, such conditions are not favorable for reversed-phase chromatographic separations because charged basic species are poorly retained on hydrophobic sorbents. Polar bases are the biggest challenge for several reasons: they are eluted in the early region of the chromatogram, where ion suppression tends to be most problematic, peak shapes are poor, and peak capacity is typically low. In high-pH mobile phases, basic compounds are ionized only partially (if at all), behaving more like neutral species. They are retained very well due to more favorable hydrophobic interaction, and are eluted with narrow and symmetrical peaks. A further benefit to our choice of mobile phase is the positive effect on the MS detection of β-blockers.

Figure 1
Instrumentation
An Agilent 1100 series (Wilmington, Delaware) HPLC system comprised of a G1312A binary pump and a G1329A autosampler module was coupled to an Applied Biosystems (Foster City, California) API 3000 triple-quadrupole mass spectrometer provided with TurboIonSpray ion source operated in positive ion mode (PIM) with multiple reaction monitoring (MRM). The source heater temperature was set to 425 °C and the heater gas flow to 7000 cc/min.
Experimental Conditions
Chromatographic separations were performed on the extended pH-range HPLC column Gemini (Phenomenex) 3 μm C18 with the dimensions 50 mm × 2.0 mm. The column was operated at a flow rate of 0.5 mL/min in gradient mode. A quantity of 5 mM ammonium bicarbonate (NH4HCO3) in water (pH 10.0) was used as mobile phase A, with acetonitrile as mobile phase B for high-pH experiments. Mobile phase A was 0.1% formic acid in water (pH 2.7), and 0.1% formic acid in acetonitrile was used as mobile phase B for low-pH experiments. Gradient elution was started at 10% B and the mobile phase composition increased to 75% B in 2.5 min. The column was reequilibrated for 1.5 min. This gradient profile was used at both low and high pH. The injection volume was 5 μL.
Sample Preparation
A 200 μL aliquot of human plasma was diluted with 500 μL of water and 100 μL of 1 M acetic acid. A 100 μL volume of 20 ng/mL oxprenolol was added as internal standard, along with 200 μL of a standard solution of β-blockers (in water) at the following concentration levels: 0.1, 0.5, 1.0, 10, 100, 500, or 1000 ng/mL, respectively. The concentration of the internal standard in the sample was 10 ng/mL.
Sample cleanup before chromatographic analysis was performed using SPE with the following procedure: strata-X-C 30 mg/1 mL cartridges (Phenomenex) were conditioned with 1 mL of methanol, followed by 1 mL of water. The sample was loaded and first washed with 1 mL of 0.1 M acetic acid solution followed by 1 mL of methanol. Analytes were eluted using 1 mL of 5% ammonium hydroxide (prepared with 28.5% concentrated ammonium hydroxide) in methanol. The extract was evaporated to dryness under gentle nitrogen gas flow and then reconstituted in 200 μL of 10% acetonitrile in water.
Results and Discussion
The Gemini 3 μm C18 110 Å high-pH stable HPLC column used in this study is well suited for the chromatographic separation of acidic, neutral, and basic compounds over an extended mobile phase pH range (1.5–12). This sorbent is made by grafting an organic protective layer onto the silica surface (9).
Previously, we reported on the successful MS detection of basic compounds in high-pH mobile phases (10). Despite the high pH reducing analyte ionization in solution (or in some cases minimizing it, such as in mobile phases of pH ≥ pKa +2), it was shown that several of the β-blockers included in the present work provided similar signal intensities in high- and low-pH mobile phases. We reconfirm these findings for all six of the β-blocker compounds examined in the previous work and report similar results for four more, at the low concentration level of 0.5 ng/mL (Figure 1). Contrary to expectation, in this and the previous work more intense signals were observed for all 10 β-blockers (including oxprenolol, the internal standard) in high-pH mobile phase, with the most significant increase in MS response observed for sotalol and atenolol (80% and 30%, respectively) at the concentration level of 0.5 ng/mL.
Figures 2 and 3 show a direct comparison of MS-MS responses at low pH (pH 2.7 in mobile phase containing 0.1% formic acid), and high pH (pH 10.0 in mobile phase containing 5 mM ammonium bicarbonate). At high pH, β-blockers are uncharged (free-base) in solution and therefore show better retention on reversed-phase sorbents. Suppressing analyte ionization effectively increases retention of the most polar β-blockers (such as sotalol and atenolol) and thus allows high-speed chromatographic separations.
Biological samples should be cleaned up before LC–MS-MS analysis for two reasons: first, for preventing the buildup of matrix components on the chromatographic column (which drastically limits its life), and second, for preventing the coelution of matrix components with analytes of interest. Such coelution often causes analyte ion suppression, which can compromise the results of the analysis and lead to incorrect quantification. The polymer-based strata-X-C (Phenomenex), an SPE sorbent possessing both hydrophobic and hydrophilic properties as well as cation-exchange capability, is effective for the selective extraction of basic compounds from complex matrices. The efficacy of the SPE procedure was evaluated at two concentration levels (0.5 and 100 ng/mL), with β-blockers spiked into human plasma. Absolute recoveries were determined by comparing MRM peak areas for standard solutions and for spiked plasma extracts at the same concentration level. Analyte absolute recoveries (for the combined SPE–LC–MS-MS assay) were >59%, while precision (RSD) was <15% (interassay; n = 6; see Table II).
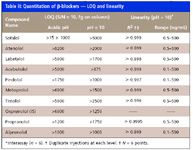
Table II
Ion-suppression experiments revealed suppression areas away from the elution region of all analytes, including early eluted compounds typically problematic in separations performed in low-pH mobile phase (Figure 4). This indicates that matrix effects can be minimized by carefully choosing the pH of the mobile phase and by extending the retention of polar compounds, which can be eluted away from the early region of the chromatogram when analyzed in uncharged form (in high-pH mobile phases).
The method employed here was linear in the concentration range 0.1–500 ng/mL. Calibration samples were prepared at the levels 0.1, 0.5, 1.0, 10, 50, 100, and 500 μg/mL in human plasma with the internal standard at 10 ng/mL. Table II shows linear regression data (R 2 ) for nine β-blockers (for SPE combined with LC–MS-MS analysis). These results demonstrate good linearity (R 2 ≥ 0.996) over a wide dynamic range (≤103 ).
The limit of quantitation (LOQ) determined as the minimum analyte amount on-column giving a signal-to-noise ratio (S/N) of 10 was 5 pg on-column or better for all β-blockers included in this study (Table III).
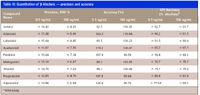
Table III
Method accuracy was evaluated by comparing the mean value of six replicate experimental results with the expected value at low and high concentration levels (0.5 and 100 ng/mL). Results are shown in Table III. Intra-assay accuracy was 85–120% at low concentration levels and 90–110% at high concentration levels.
Conclusions
A high-speed reversed-phase LC–ESI-MS-MS method was developed for the analysis of nine β-blockers using SPE cartridges for sample cleanup, and a C18 HPLC column for chromatographic separation within 4 min (total cycle time).

Figure 2
The β-blockers included in this study were detected with good sensitivity in high-pH mobile phase (pH 10.0) containing ammonium bicarbonate buffer and acetonitrile.
Significant matrix effects for polar β-blockers can be minimized by choosing the mobile phase pH judiciously; increased retention of polar compounds (such as polar bases in high-pH mobile phase) will move their retention time away from the early region of intense ion suppression.
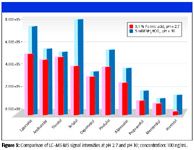
Figure 3
The simultaneous quantitation of β-blockers by LC–ESI-MS-MS in a mobile phase containing a high-pH buffer provides an attractive alternative for analyzing and monitoring β-blockers in biological samples in diagnostics, therapeutic monitoring, doping screening, and forensic analysis.

Figure 4
References
(1) J.M. Cruickshank and B.N.C. Prichard, Beta-blockers in Clinical Practice (Churchill Livingstone Inc., New York, 1988).
(2) M. Schulz and A. Schmoldt, Pharmazie 58, 447 (2003).
(3) WADA. The World Anti-Doping Code. The 2004 Prohibited List, International Standard, 2004.
(4) H. Ktaoka, S. Narimatsu, H.L. Lord, and J. Pawliszyn, Anal. Chem.71, 4237–4244 (1999).
(5) M.E. Abdel-Hamid, Farmaco 55, 136–145 (2000).
(6) K. Deventer, P. Van Eenoo, and F.T. Delbeke, Rapid Commun. Mass Spectrom. 19, 90–98 (2005).
(7) R.D. Johnson and R.J. Lewis, Forensic S. International 156, 106–117 (2006).
(8) M. Josefsson and A. Sabanovic, J. Chromatogr. A. 1120, 1–12 (2006).
(9) US Patent 2,005,035,217 (September 30, 2005).
(10) L. Peng and T. Farkas, J. Chromatogr. A.1179, 131–144 (2008).
Liming Peng and Tivadar Farkas are with Phenomenex Inc., Torrance, California.


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










