High Performance Mass Spectrometry for Small Molecule and Protein Applications
Special Issues
The author discusses the use of high-performance mass spectrometry for small molecule and protein applications.
For many years, and after several notable failures, many researchers were convinced that it was impossible to design a quadrupole time-of-flight (qTOF) mass spectrometer that was able to retain its ability to perform the high resolution measurements necessary for definitive molecular formula determination of unknowns. Conventional wisdom indicated that there were many reasons (such as temperature stability, ion diffusion, and ion loss on grids of reflectrons) that would make it impossible to improve resolution of these types of instruments. Figure 1 shows the internal layout of a TOF instrument designed to overcome the limitations of mass spectrometry (MS) with fast chromatography. The diagram shows improvements such as the inclusion of an ion chiller, the use of a series of ion refocusing operations, the presence of a single reflectron to minimize ion losses, and temperature control of the instrument's overall flight tube to minimize diffusion of ions.
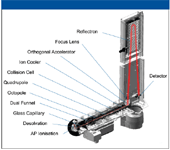
Figure 1: Schematic of the internal design of the maXis UHR-TOF mass spectrometer (Bruker Daltonics).
Initial reports of the instrument's performance in both MS and MS-MS mode indicate that it is able to maintain resolution beyond 40,000 at speeds of up to 20 Hz. The mass accuracy is reported to be < 1 ppm. The mass range is 50–20,000 m/z, It is designed to provide a dynamic range of nearly five orders of magnitude dynamic range.
The following section describes a series of experiments for small molecule and protein analysis that were designed to test MS performance.
MS with Fast Chromatography and Small Molecules
A series of five unrelated drug compounds were chromatographed using a reversed-phase column and a 30-s elution gradient. The results of the initial studies are presented in Figure 2. As can be seen, the method was able to achieve baseline separation of the five compounds in 30 s. In addition, the peak widths, when measured at half height, were all less than 1 s.
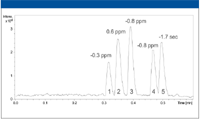
Figure 2: Separation and measurement of drug compounds with fast chromatography. Peaks: 1 - norharmane, 2 - sulfamethazine, 3 - sulfamethazole, 4 - oxybutynin, 5 - terfenadine.
Figure 3 is a closer examination of the fourth peak in the compound series, oxybutynin. The left half of the figure shows the extracted ion chromatogram showing that more than 20 data points were collected fora 1-s peak. This leads to the high levels of both mass accuracy (0.8 ppm) and resolution (> 40,000) shown in the right half of the figure. Other peaks showed similar results.
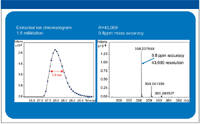
Figure 3: Close examination of a single fast chromatography peak.
MS with Intact Proteins
As many groups are focusing more and more on protein and peptide analysis, effective analysis of intact proteins has become increasingly important. Figure 4 illustrates the data obtained when measuring a sample of intact IgG antibody.
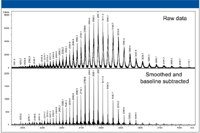
Figure 4: MS measurement of intact IgG.
The data shown here indicate that with intact proteins as large as IgG (148 kDa), high resolution data can be obtained to further analyze large, commercially important molecules that generate high m/z species, such as antibodies. With this resolution, it becomes possible to directly analyze the glycoforms of intact proteins such as IgG (Figure 5).
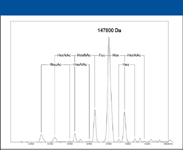
Figure 5: MS analysis of the glycoforms of intact IgG.
This study yielded high resolution data for intact proteins and specific information about the minute differences in proteins found in different glycosylation patterns. The high resolution capability demonstrated here would be extremely useful for rapid profiling of a series of proteins or peptides in a biotherapeutics environment, for instance.
Conclusion
Today's analytical chemistry environment demands the deployment of more sophisticated methods and instrumentation to keep pace with the profound changes in separation techniques being adopted by many laboratories. Two of the most important tools for the future will be fast chromatography and mass spectrometry.
Darwin Asa is Marketing Manager, Bruker Daltonics, Billerica, Massachusetts.

Altering Capillary Gas Chromatography Systems Using Silicon Pneumatic Microvalves
May 5th 2025Many multi-column gas chromatography systems use two-position multi-port switching valves, which can suffer from delays in valve switching. Shimadzu researchers aimed to create a new sampling and switching module for these systems.
Studying Cyclodextrins with UHPLC-MS/MS
May 5th 2025Saba Aslani from the University of Texas at Arlington spoke to LCGC International about a collaborative project with Northwestern University, the University of Hong Kong, and BioTools, Inc., investigating mirror-image cyclodextrins using ultra-high performance liquid chromatography–tandem mass spectrometry (UHPLC–MS/MS) and vibrational circular dichroism (VCD).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










