High Efficiency Chiral Separations in HPLC and SFC
LCGC North America
In advance of PittconIn advance of Pittcon 2018, leading scientists-Ronald Majors, Richard Henry, John W. Dolan, Zachary S. Breitbach, and Daniel W. Armstrong-who will be speaking at the LCGC awards symposium give us a preview of their talks.
For the past three decades, research and development of new chiral stationary phases (CSPs) has focused on the evaluation and optimization of novel or improved chiral selectors. This focus has been very important because it is well known that maximizing selectivity is the most effective way to improve resolution of two enantiomers. However, it also has led to a shortage of other technological advancements for new CSPs. For example, usual efficiencies of 5-µm fully porous particle (FPP)-based chiral columns are typically an order of magnitude lower than the efficiencies of state-of-the-art achiral columns (that is, 25,000–50,000 plates/m for chiral columns versus 250,000–350,000 plates/m for achiral columns). It is clear that improvements in chiral column technology have lagged behind innovative, high efficiency achiral phases. Herein, recent research on high efficiency chiral stationary phases is described.
Narrow Particle Size Distribution Sub-2-µm Chiral Stationary Phases
The van Deemter equation promises increased efficiency with decreases in particle size. Further, if smaller particles can also be made more uniformly and packed better, then there is an opportunity to achieve very highly efficient columns and chromatography. Using 1.9-µm FPPs that possessed a very narrow particle size distribution (NPSD), the macrocyclic chiral selectors vancomycin, teicoplanin, and teicoplanin aglycone were bonded to sub-2-µm FPPs (1). Interestingly, the surface area of these particles is similar to 5-µm FPPs used in commercial macrocyclic glycopeptide columns. As such, the surface coverage of the chiral selectors on the sub-2-µm FPPs was found to be very similar to commercial 5-µm FPP-based chiral phases. An example of the improved efficiency that can be obtained using a 1.9-µm NPSD FPP CSP is shown in Figure 1a. The clear gains in efficiency are evident as the plate number for the first eluted enantiomer increased more than four times going from the 5-µm column to the 1.9-µm NPSD column. Additionally, the resolution also increased (approximately doubled) on the 1.9-µm NPSD-based column. Also worth noting is the identical retention and selectivity values between the 5-µm and 1.9-µm columns (Figure 1a), meaning one can directly convert back and forth between ultrahigh-pressure liquid chromatography (UHPLC) to high performance liquid chromatography (HPLC)-based chiral phases. Finally, when the 1.9-µm NPSD FPP-based chiral column was compared to an analogous 1.7-µm FPP column (not specifically engineered to control PSD); the NPSD column was twice as efficient at an optimum flow rate (1). The NPSD column also proved more permeable than the standard 1.7-µm FPP column. The high efficiency and reasonable permeability of the NPSD column allowed for the evaluation of ultrafast separations (that is, occurring in less than 60 s) at high flow rates using short columns. Figure 1b shows two examples of ultrafast chiral separations. Of course, as with any small particles, the use of high flow rates leads to increased system pressure, but this is less of an issue in supercritical fluid chromatography (SFC) chiral separations.
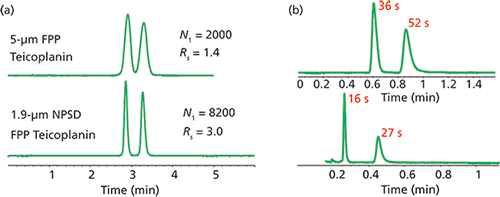
Figure 1: (a) Enantiomeric separation of propranolol on teicoplanin-based chiral columns and (b) ultrafast enantiomeric separation of m-tyrosine (top) and thalidomide (bottom) on the 1.9-µm NPSD FPP teicoplanin CSP. Conditions (a): mobile phase: 60:40:0.3:0.2 acetonitrile-methanol-AA-triethylamine; flow rate: 1.0 mL/min, temperature: 22 °C. Conditions (b), top: mobile phase: 70:30 water-methanol; flow rate: 1.5 mL/min, temperature: 22 °C. Conditions (b), bottom: mobile phase: methanol; flow rate: 3.0 mL/min; temperature: 22 °C.
Superficially Porous Particle–Based Chiral Stationary Phases
An alternative way to improve chiral column efficiency is through the use of superficially porous particles (SPPs). This approach is particularly attractive since SPP-based achiral HPLC columns (~3 µm) have been known to yield plate counts as high as sub-2-µm columns without the consequent high pressures.
The first successful brush-type SPP based CSPs were made by immobilization of cyclic oligosaccharides such as cyclofructans and cyclodextrins (2,3). Figure 2a shows an example of a chiral separation on a cyclodextrin (hydroxypropyl-β-cyclodextrin [HPBCD]) SPP-based column compared to 5-µm (that is, the commercially available column) and 3-µm FPP columns under constant mobile phase conditions (that is, all separations were performed using the exact same mobile phase). As can be seen the selectivity values on the three columns in Figure 2a are similar with the SPP CSP resulting in the highest alpha value of 1.33. This result was pivotal to the success of SPP-based CSPs. Since SPPs inherently have less surface area than FPPs, SPP-based CSPs will exhibit overall lower carbon load per mass of packing material compared to FPPs. However, when the carbon load is normalized for surface area, SPP-based phases always have equal or slightly higher effective coverages compared to FPPs. The result of this higher coverage is that selectivity does not suffer and is sometimes even improved on the lower surface area SPP-based CSPs. Also, in Figure 2a it is shown that the SPP-based column resulted in about three times the efficiency of a commercial 5-µm FPP column and two times the efficiency of an analogous 3-µm FPP column. The resolution value on the SPP column is certainly improved compared to the 5-µm FPP-based column, but slightly lower than the 3-µm FPP column. The reason the 3-µm column is slightly better is the increased retention on the FPP-based columns, which is a result of the greater surface area. As such, a second comparison of the three phases was made in Figure 3b. In this comparison, the mobile phases were adjusted so that each column exhibited a constant analysis time. Here it is clear that the SPP-based column produced the greatest separation and is the only column that is able to resolve the hydrobenzoin enantiomers within the 2-min run-time.
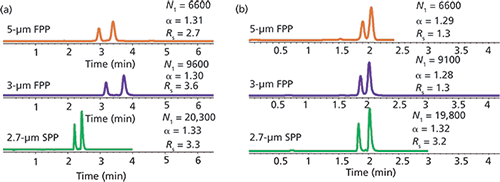
Figure 2: (a) Enantiomeric separation of R,R/S,S-hydrobenzoin on hydroxypropyl-β-cyclodextrin bonded to FPPs and SPPs. Conditions (a): mobile phase (constant mobile phase mode): 75:25 10 mM ammonium acetate, pH 4.1-acetonitrile; flow rate: 1.0 mL/min. Conditions (b): mobile phase (constant elution mode): 10 mM ammonium acetate, pH 4.1-acetonitrile at ratios 64:36 (top), 62:38 (middle), and 75:25 (bottom); flow rate: 1.2 mL/min.
When the kinetic performance of the SPP-based CSP was evaluated, it was found that increasing the flow rate does not negatively affect the separation to the extent that it does when the flow rate is increased on FPP-based columns. For example, when using a cyclofructan-based CSP at a flow rate of 3.0 mL/min, the number of plates on column afforded by the SPP column was seven times greater than the number of plates on column (same length) obtained when using a commercial 5-µm FPP-based column. Given their high efficiencies and relatively low back pressures, columns containing these SPP particles were particularly advantageous for ultrafast chiral separations in the 4–40 s range (4).
Conclusions
Using sub-2-µm NPSD FPPs and 2.7-µm SPPs, high efficiency chiral stationary phases have been developed. When properly packed these columns can produce >200,000 plates/m and nearly reach the efficiency of achiral columns. Both types of high efficiency CSPs can be used to produce ultrafast enantiomeric separations, but it is easier to achieve higher flow rates within acceptable pressure ranges using the SPP-based columns in HPLC. More recently, SPP CSPs have been used to produce sub-second chiral separations (5) and even the separation of multiple (up to 10) components in less than 1 s. Several SPP-based chiral phases are now commercially available and are likely to replace their analogous traditional 5-µm FPP-based columns in time. As an aside, it is also worth noting that native cyclofructan immobilized on SPPs was also evaluated as a hydrophilic interaction chromatography (HILIC) stationary phase, resulting in a highly efficient and-even more important-highly stable HILIC column with very unique selectivity (6). For a more detailed overview of high throughput and high efficiency chiral separations see our recent review in the Journal of Chromatography A (7).
References
(1) U.C.L. Barhate, M.F. Wahab, Z.S Breitbach, D.S. Bell, and D.W. Armstrong, Anal. Chim. Acta 898, 128–137 (2015).
(2) D.A. Spudeit, M.D. Dolzan, Z.S. Breitbach, W.E Barber, G.A. Micke, and D.W. Armstrong, J. Chromatogr. A 1363, 89–95 (2014).
(3) D.A. Spudeit, Z.S. Breitbach, M.D. Dolzan, G.A. Micke, and D.W. Armstrong, Chirality 27, 788–794 (2015).
(4) D.C. Patel, Z.S. Breitbach, M.F. Wahab, C.L. Barhate, and D.W. Armstrong, Anal. Chem. 87, 9137–9148 (2015).
(5) M.F. Wahab, R.M. Wimalasinghe, Y. Wang, C.L Barhate, D.C. Patel, and D.W. Armstrong, Anal. Chem. 88, 8821–8826 (2016).
(6) M.D. Dolzan, D.A. Spudeit, Z.S. Breitbach, W.E. Barber, G.A. Micke, and D.W. Armstrong, J. Chromatogr. A 1365, 124–130 (2014).
(7) D.C. Patel, M.F. Wahab, D.W. Armstrong, and Z.S. Breitbach, J. Chromatogr. A 1467, 2–18 (2016).
Zachary S. Breitbach

Zachary S. Breitbach is with AbbVie Inc., Research & Development in North Chicago, Illinois. He is also the recipient of LCGC's 2018 Emerging Leader in Chromatography Award.

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













