"Green" HPLC with 100% Water Eluent for Analysing Melamine in Milk
LCGC Europe
A novel "green chromatography" method for analysing melamine in milk is described. The method uses 100% water as the eluent and in the sample preparation stage and is reported to be a reliable and cost-effective method for laboratories in developing countries.
A reversed-phase (RP) high performance liquid chromatography (HPLC) method for analysing melamine residue in cow's milk using 100% water in the sample preparation stage and as the eluent is described. HPLC was performed on an ODS column with an isocratic 100% water mobile phase and a photo-diode array (PDA) detector. The proposed method was validated by the analyses of spiked milk samples resulting in recoveries greater than 96.8% with a relative standard deviation (RSD) of less than 2.0%. The total analysis time was less than 15 min/sample and the quantitation limit was 0.05 µg/g.

Melamine [1,3,5-triazine -2,4,6-triamine (C3H6N6)] is an inexpensive nitrogen-containing industrial chemical that is widely used in the manufacture of plastics and adhesives, but can also be used to alter tests to check the protein content of milk products (Figure 1). Adding melamine increases the nitrogen content of the milk and its apparent protein content measurement, which falsely increases the final price of the milk.
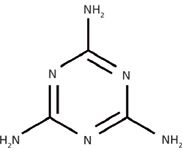
Figure 1: Chemical structure of melamine.
Recent reports from several countries on food products containing milk contaminated with melamine have led to an increased interest in the analysis of this compund. Melamine contamination has been detected in various food products containing milk (1–3). Significant consumption of melamine can result in renal failure and, in rare cases, death. In fact, melamine contamination in milk products has resulted in illness in nearly 300,?000 babies and the deaths of at least six infants in China in 2008 (3–5).
The European Union set a standard for acceptable human consumption (the tolerable daily intake — TDI) of melamine at 0.5 milligrams per kg of body mass (reduced to 0.2 mg per kg in April 2010) (6,7). Canada declared a limit of 0.35 mg and the US Food and Drug Administration's (FDA's) limit was put at 0.63 mg, but was later reduced to 0.063 mg daily. The World Health Organization's (WHO's) food safety director estimated that the amount of melamine a person could stand per day without incurring a bigger health risk was a TDI of 0.2 mg per kg of body mass (6,8). The Codex Alimentarius Commission has set the maximum amount of melamine allowed in food products containing milk to 2.5 µg/g and the amount of the chemical allowed in powdered infant formula to 1 µg/g (9), equivalent to the FDA's safety limit (3). Milk is an indispensable food and a base raw material of various foods because it is highly nutritious, inexpensive and readily available. Strict monitoring of the presence of melamine in milk is, therefore, an important activity as a means of guaranteeing food safety.
The development of international standardized methods to determine chemical residues in foods is essential to guarantee equitable international trade in these foods and ensure food safety for consumers. Whether in industrial nations or developing countries, an internal harmonized method for residue monitoring in foods is needed. The optimal method for the routine monitoring of chemicals in foods should be simple, quick, economical in time and cost and, importantly, cause no harm to the environment and analyst.
Eliminating the use of organic solvents and reagents is an important goal in terms of environmental conservation, human health and the economy.
The reported methods for quantification of melamine in milk include enzyme-linked immunosorbent assay (ELISA) (10), GC–MS (10,11), HPLC–UV (12) or diode array detector (DAD) (13) and LC–MS–MS (14,15). The FDA issued new methods for the analysis of melamine in liquid formula, based on LC–MS–MS detection (16). The direct analysis of liquid milk by low-temperature plasma MS (LTP–MS) (17) and ultrasoundassisted extractive electrospray ionization (EESI–MS) (18) have recently been proposed. The facilities that GC–MS, LC–MS–MS, LTP–MS or EESI–MS system are available in are limited because these methodologies use complex, specific and expensive instrumentation. These are unavailable in a lot of laboratories for routine analysis, particularly in developing countries. All of the methods mentioned above consume organic solvents and/or reagents. No optimal method that satisfies the requirements for melamine analysis previously described has yet been identified.
This article describes a safe and simple method to monitor melamine residue in milk using 100% water in sample preparation and HPLC separation without using organic solvent and reagents.
Experimental
Materials: Distilled water (HPLC grade) and melamine standard were purchased from Wako Pure Chem. Ltd. (Osaka, Japan). A stock melamine standard solution of 100 µg/mL in water was taken into a cryo-vial, sealed and refrigerated until further use. Working standard solutions were prepared by diluting the stock solution with water.
The following apparatus were used in the sample preparation stage: handheld ultrasonic-homogenizer (model HOM100, 2 mm i.d. probe, Iwaki Glass Co., Ltd., Funabashi, Japan); micro-centrifuge (Biofuge fresco, Kendo Lab. Products, Hanau, Germany); MonoSpin C18 as a centrifugal monolithic silica spin mini-column (GL Sciences Inc., Tokyo, Japan) (highly pure sol-gel silica gel monolith, sample throughput volume ≤ 300 µL). The spin column (GL Sciences) was pre-conditioned as follows: 100 µL of water was added and then centrifuged at 3,500 g for 1 min at room temperature.
HPLC: The following types of non-polar sorbent columns (5 µm dp, 250 or 150 × 4.6 mm) for HPLC were used: Wakosil 5TMS (C1) (Wako); Mightysil RP-4GP (C4) (Kanto Chemical Co., Inc., Tokyo, Japan); Lichrospher 60 RP-selectB (C8) (Merck, Darmstadt, Germany); Mightysil RP-18 GP Aqua (C18) (Kanto); Mightysil RP-18 (C18) (Kanto); Inertsil HILIC (alkyl diol) (GL Sciences, Tokyo, Japan); Inertsil ODS-4 (C18) (GL Sciences) (Table 1).

Table 1: Physical/chemical specifications of the reversed-phase columnsa used and chromatographic melamine separation obtained under the HPLC conditions examinedb.
The HPLC system included a model PU-980 pump and DG-980-50 degasser (both from Jasco Corp., Tokyo, Japan), as well as a model SPD-M10A VP diode array detector (DAD) (Shimadzu Scientific Instruments, Kyoto, Japan). The analytical column was an Inertsil ODS-4 (octadecyl chemically bonded silica, C18) (250 × 4.6 mm, 5 µm dp, 450 m2 g-1 surface area, 100 Å pore diameter, 1.05 mL/g pore volume, 11% carbon load) column (GL Sciences) using a water mobile phase at a flow rate of 1.0 mL min-1 at room temperature. The 25 °C DAD was operated at 190–300 nm: monitoring the wavelength for melamine was adjusted to 202 nm which is an absorption maximum for the analyte. The injection volume was 20 µL.
Sample preparation: An accurate 0.1 mL sample was taken into a micro-centrifuge tube and homogenized with 0.6 mL of water with a handheld ultrasonic-homogenizer for 30 s. The capped tube was then centrifuged at 10000 g for 5 min. 100 µL of supernatant liquid was poured into a MonoSpin C18 mini-column and centrifuged at 3,500 g for 1 min. The eluate was injected into the HPLC system.
Results and Discussion
Sample Preparation: The ultrasonic-homogenization allowed the satisfactory extraction of melamine from samples with 100% water. The extract obtained was purified by subsequent centrifugal monolithic silica spin minicolumn: MonoSpin C18. The mini-column is a monolithic SPE column which is said to be excellent for small volume samples with easy and quick operation by centrifuge (19).
The monospin method is not only used for deproteinization but can also be used for defatting. Since the recovery of the sample from the column varies with centrifugal acceleration (rotary speed), we investigated the optimum acceleration (> 1,000 g) to recover melamine from the spin mini-column.
In this test, blank milk sample extract processed with water was centrifuged as described earlier. The supernatant was then fortified with two-level melamine standard solutions and mixed. A 100 µL portion of the sample extract containing 0.25 µg or 0.5 µg of melamine was applied to the spin minicolumn. The centrifugal time was standardized at 1 min. The optimal acceleration that recovered melamine from MonoSpin C18 was at 3,500 g.
There were no significant differences in data between two spiking levels (2.5 µg/mL and 5 µg/mL, n = 5 for each). The purification treatment by MonoSpin at 3,500 g for 1 min gave a satisfactory recovery (> 98 %) and reproducibility (RSD = 1.3 %, n = 10) for melamine. The time required for the sample preparation of a single sample was < 8 min. For sequential analyses, a batch of 24 samples could be prepared in 40 min. The eco-friendly procedure provided an easy-to-use and efficient purification and had a short operation time, resulting in high repeatability (Table 2) and great saving analysis costs.
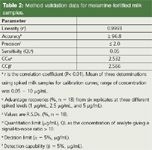
Table 2: Method validation data for melamine-fortified milk samples.
Optimal HPLC Conditions: In order to achieve separation with a 100% water mobile phase, this study tested eight types of non-polar sorbent columns. The physical and chemical specifications are listed in Table 1. We used 100% water as an isocratic mobile phase and examined column temperatures (≥20 °C) and flow rates (≥ 0.5 mL/min). The eight columns were compared and judged on how they separated between melamine and its interfering peaks and the sharpness of melamine peak obtained upon injection of equal amounts. The chromatographic separations obtained under these conditions are also presented in Table 1.
Columns A–F had difficulty separating melamine and the interfering milk extract throughout the examined conditions. They separated melamine as a broadening or rounded peak. The complete separation of melamine and the interference peaks and its symmetrical peak was achieved with Column H using an isocratic mobile phase of water with a column temperature of 25 °C and a flow-rate of 1.0 mL/min.
The melamine peak is clearly distinguished at 6.3 min (Figure 2). The present HPLC analysis accomplished optimum separation in a short time without the need for a gradient system to improve the separation and pre-column washing after an analysis.
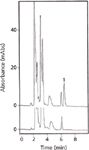
Figure 2: Chromatograms obtained from the present HPLC system. A milk sample spiked with melamine (1 �g/mL) (upper profile) and a blank milk sample (lower profile). Peak: 1, melamine (retention time = 6.3 min).
Figure 2 shows that the resulting chromatograms were free from interfering compounds for quantitation and identification of melamine by HPLC, with the DAD set at 202 nm (giving the maximum absorbance for melamine).
Method Validation
Main parameters: The main validation parameters are summarized in Table 2. The linearity, accurary, precision and quantitation limit (QL) are well within acceptable criteria for the residue analysis that the Codex set up (20). The decision limit (CCα, α = 5%) and detection capability (CCβ, β = 5%) calculated in accordance with the EU regulation decision (2002/657/EU) are also displayed in this table. In the CCα and CCβ calculations, the Codex's maximum amount of melamine, 2.5 µg/mL in milk, equivalent to the FDA's safety limit (3,9), was taken as an established permitted limit (21). The other parameters are as follows:
Selectivity: The present HPLC system easily confirmed the peak identity of the target compound. Melamine was identified in the sample by its retention time and absorption spectrum. The separation, identification and quantification were achieved in a mere 6–7 min run without requiring a gradient system to improve the separation or pre-column washing after an analysis. The HPLC system did not need to use MS–MS–MS to identity the target compound.
Robustness: The robustness of the HPLC system was examined by using a spiked (2.5 µg/mL of melamine) milk sample obtained under the established procedure.
Changes of ±5% units of the two chromatographic parameters, flow rate (1.0 mL/min) and column temperature (25 °C) were determined. The effects on the peak area and the validations in the retention time were evaluated. Changes of ±5% of the flow rate and the column temperature had no effect on the peak area, whereas the slight variations in the retention time were obtained with both parameters. Normal retention time for melamine was 6.3 min. At +5% flow rate, the retention time was decreased, and at –5% the time was increased. The decreasing and increasing retention times were 1.5 and 5.1%, respectively. By changing the column temperature by +5%, a decreasing retention time of 5.4% was obtained, however, no significant variations were observed with –5%. During these studies the target compound was separated.
Cost and time performance: The time and budget required for the analysis of one sample were < 15 min and roughly 4.72 Euro (¥500, as on 2 May 2012), respectively. These findings became a criteria in routine residue analysis.
Conclusions
The present procedure was easy-to-use, rapid, spacesaving, and did not involve the use of organic solvents/reagents. It also resulted in high recovery and reproducibility with considerable saving of analysis time/cost. The procedure may be proposed as an international standardized method for routine residue monitoring of melamine in milk.
Acknowledgment
The authors thank Mr Yoshimura (GL Sciences) for supplying MonoSpin C18 spin mini-column and Inertsil ODS-4 column.
Naoto Furusawa (Doctor of Agriculture) is an associate professor with the Graduate School of Human Life Science, Osaka City University, Osaka, Japan.
Eriko Tsumatani is a food sanitation supervisor with the Okamura Oil Mill Co., Ltd., Kawahara, Osaka, Japan.
References
(1) World Health Organization, Melamine contamination event, China, 2008, retrieved 2 December 2008, from http://www.who.int/foodsafety/fs management/infosan events/en/index.html
(2) World Health Organization, Melamine and Cyanuric acid: Toxicity, Preliminary Risk Assessment and Guidance on Levels in Food, 25 September 2008; updated 30 October 2008, from http://www.who.int/foodsafety/fs_management/Melamine.pdf
(3) HUFFPOST TECHNOLOGY, UN food safety meeting sets melamine limit, 6 July 2010, from http://www.huffingtonpost.com/huff-wires/20100706/un-un-melamine-limit/
(4) E.Y. Chan, S.M. Griffiths and C.W. Chan, Lancet, 372(9648), 1444–1445 (2008).
(5) M.S. Filigenzi, B. Puschner, L.S. Aston and R.H. Poppenga, J. Agric. Food Chem., 56(17), 7593 (2008).
(6) Wikipedia, from http://en.wikipedia.org/wiki/Melamine#cite_note-67
(7) Rory Harrington (15 April 2010), "EFSA cuts melamine TDI by 60 per cent". FoodQualityNews.com, from http://www.foodqualitynews.com/Legislation/EFSA-cuts-melamine-TDI-by-60-per-cent?utm_source=RSS_text_news. Retrieved 2010-04-16.
(8) Lara Endreszl (10 December 2008). "Safe Melamine Levels Named by World Health Organization". Health News, from http://www.healthnews.com/alerts-outbreaks/safe-melamine-levels-named-world-health-organization-2252.html
(9) World Health Organization. 2010-07-06, "International experts limit melamine levels in food", from http://www.who.int/mediacentre/news/releases/2010/melamine_food_20100706/en/index.html
(10) P. Lutter, M.C. Savoy-Perroud, E. Campos-Gimenez, L. Meyer, T. Goldmann, M.C. Bertholet, P. Mottier, A. Desmarchelier, F. Monard, C. Perrin, F. Robert and T. Delatour, Food Control, 22(6), 903–913 (2011).
(11) X.L. Zhu, S.H. Wang, Q. Liu, Q. Xu, S.X. Xu and H.L. Chen, J. Agric. Food. Chem., 57(23), 11075–11080 (2009).
(12) G. Venkatasami and J.R. Sowa Jr., Anal. Chim. Acta, 665(2), 227–230 (2010).
(13) M. Rambla-Alegre, J.Peris-Vicente, S. Marco-Perio, B. BeltranMartinavarro and J. Esteve-Romero, Talanta, 81(3), 894–900 (2010).
(14) J. Mi, Z. Zhang, Z. Zhang, P. Li and Y. Fang, Agilent Technologies Application Note 5989-9950EN, Oct 2008.
(15) B. Tran, R. Okoniewski, R. Storm, R. Jansing and K.M. Aldous, J. Agric. Food. Chem., 58(1), 101–107 (2010).
(16) U.S. FDA Laboratory Information Bulletin No 4421, from http://www.cfsan.fda.gov/~frf/lib4421.html
(17) G. Huang, Z. Ouyang and R.G. Cooks, Chem Commun., 5(5), 556–558 (2009).
(18) L. Zhu, G. Gamez, H. Chen and K. Chingina, Chem. Commun., 5(5), 559–561 (2009).
(19) GL Sciences, from http://www.glsciences.com/products/monolithic_products/mono_spin.pdf
(20) Codex Alimentarius Commission, Joint FAO/WHO Food Standards Program, from http://www.maff.go.jp/j/syouan/kijun/codex/pdf/manual_19e.pdf#search='Codex alimentarius Residues of veterinary drugs Rome'
(21) 2002/657/EC: Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (Text with EEA relevance) (notified under document number C(2002) 3044), from http://eur-lex.europa.eu/smartapi/cgi/sga_doc?smartapi!celexapi!prod!CELEXnumdoc&lg=EN&numdoc=32002D0657&model=guichett
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.









