GPC/SEC–MALLS Analysis of Hyaluronic Acid
PSS Application Note
Introduction
Hyaluronic acid is a polymer of disaccharides and can have up to 25000 disaccharide repeat units. The molar mass range is from 5000 Da to 20000000 Da. Hyaluronic acid is mainly used in medical and cosmetic applications.
GPC/SEC is the method of choice for measuring the molar mass distribution of hyaluronic acid. True molar masses can be obtained when online multi angle light scattering is used in combination with a refractive index detector. The refractive index increment (dn/dc) for light scattering data evaluation can be determined online, measured offline using dedicated instrumentation, or taken from literature.
Experimental Conditions
Eluent: 0.005 M phosphate buffered saline, 0.069 M NaCl
Columns: PSS SUPREMA Lux 10 µm 30Å, 10 000Å and 10000Å (8 × 300 mm) + precolumn
Data acquisition: PSS WinGPC Unity with Compliance Pack
Detectors: SECcurity GPC1200 RI SECcurity MALLS SLD7000
Flow-rate: 0.5 mL/min
Concentration: 0.5 g/L
Injection volume: 100 µL
Results and Conclusions
High molar mass samples require lower flow-rates and concentrations in GPC/SEC. The GPC/SEC conditions and columns have been optimized for high molar mass hyaluronic acid with respect to loading, flow-rate, column particle size and porosity. For sample preparation the water content of approx. 12% has been taken into account.
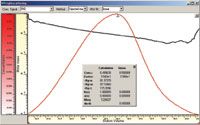
Figure 1: Slice concentration and molar mass obtained with a SLD7000 and an RI detector.
Due to the lack of high molar mass calibration standards the use of light scattering detection is recommended. This allows also to measure true molar masses.
A refractive index increment (dn/dc) for hyaluronic acid of 0.165 mL/g has been used to evaluate the light scattering data.1 Figure 1 shows the slice concentration measured by the RI detector as well as the online measured molar mass. Sample recovery (compare Conc. Calculation vs Given) was nearly 100% showing that all material eluted from the column.

Figure 2: Molar mass distribution, molar mass averages and cumulative distribution for a hyaluronic acid obtained using GPC/SEC-MALLS.
Table 1 shows the molar mass averages of the hyaluronic acid sample obtained using a Pullulan calibration (RI only, apparent molar masses) and using light scattering. The light scattering Mw molar mass is three times lower than that obtained with a pullulan calibration curve.

Table 1: Comparison molar mass averages: Only light scattering gives absolute molar masses.
References
1. Lavrenko, Linow and Gornitz in Analytical Ultracentrifugation in Biochemistry and Polymer Science, Royal Society of Chemistry, Cambridge, 517–531 (1992).
PSS Polymer Standards Service GmbH
In der Dalheimer Wiese 5, D-55120 Mainz, Germany
tel. +49 6131 962390 fax +49 6131 96239 11
E-mail: info@polymer.de
Website: www.polymer.de

Discovering the Hidden Plastic in Garden Compost with Pyr-GC–MS
May 12th 2025An Australian study used pyrolysis coupled to gas chromatography-mass spectrometry (Pyr-GC–MS) to analyze the presence of plastic polymers in commercial and homemade composts. LCGC International spoke to Simran Kaur—a PhD candidate at the Queensland Alliance for Environmental Health Sciences (QAEHS) at The University of Queensland in Woolloongabba, Australia—to find out more about her team’s findings.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














