An Improved SPE-LC–MS–MS Platform for the Simultaneous Quantification of Multiple Amyloid Beta Peptides in Cerebrospinal Fluid for Preclinical or Biomarker Discovery
Waters Application Note
Introduction
Fast, flexible platforms for peptide quantification are needed, particularly for a discovery setting. This type of methodology would be especially advantageous in the case of amyloid beta (aβ) peptides. The deposition/formation of insoluble aggregates, or plaques, of aβ peptides in the brain is considered to be a critical event in the progression of Alzheimer's disease (AD) and thus has the attention of many researchers. A previous Waters application note (720003682en) described in detail the development of a fast, flexible SPE-LC–MS–MS platform for the quantification of multiple aβ peptides from human or monkey CSF for use in a biomarker or preclinical discovery setting. In this work, the mass spectrometry platform has been updated from the Xevo TQ MS to the Xevo TQ-S mass spectrometry system. This change facilitated both a 4× reduction in required sample size and a 4–5× increase in assay sensitivity. This work focuses on methods for the 1-38, 1-40 and 1-42 aβ, Table 1.

Table 1: Sequence, MW and pI information for amyloid peptides.
Experimental Conditions
SPE-LC–MS–MS Conditions
LC system: Waters ACQUITY UPLC System
Column: ACQUITY UPLC BEH C18 300 Å, 2.1 × 150 mm, 1.7 µm, Peptide Separation Technology
SPE: Oasis MCX µElution 96-well plate, 50 µL human or animal CSF
MS system: Waters Xevo TQ-S, ESI+

Figure 1: Representative ESI+ MS-MS spectrum for amyloid 1-42 with fragment sequence ions labelled.
Results and Conclusions
• An improved SPE-UPLC–MS–MS bioanalytical method was developed and validated for the simultaneous quantification of multiple amyloid β peptides in human CSF.
• MS was performed in positive ion mode since CID of the 4+ precursor ion yielded several distinct product ions corresponding to inherently specific b sequence ions (representative spectrum shown in Figure 1).
• UPLC separation of the three amyloid β peptides is shown in Figure 2.
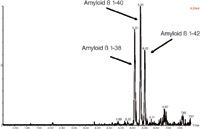
Figure 2: Representative UPLCâMSâMS analysis of amyloid 1-38, 1-40 and 1-42 peptides extracted from artificial CSF + 5% rat plasma.
• The increased sensitivity of the Xevo TQ-S triple quadrupole mass spectrometer facilitated the use of 4× less sample and a 4–5× improvement in quantification limits (Table 2).
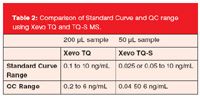
Table 2: Comparison of Standard Curve and QC range using Xevo TQ and TQ-S MS.
• Average basal levels and RSD values for all 3 aβ peptides in 2 sources of human CSF are shown in Table 3 and are lower or equal to 5%.
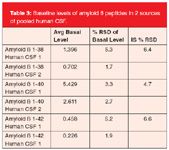
Table 3: Baseline levels of amyloid peptides in 2 sources of pooled human CSF.
• Overspiked QC samples were prepared in triplicate in 2 sources of pooled human CSF at 0.04, 0.075, 0.15, 0.2, 0.8, 2 and 6 ng/mL. Accuracy and precision values met the regulatory criteria for LC–MS–MS assays. Results from QC sample analysis are shown in Table 4. Average deviation from expected is 2.3%
The method described herein eliminates time-consuming immunoassays or immunoprecipitation steps for pre-clinical work.

Table 4: Average deviation values for all overspike QC samples
The use of a single UPLC–MS–MS assay represents a significant advantage over an ELISA assay, which would require multiple assays with multiple antibodies to quantify each of the relevant peptides.
Copyright 2010 : ACQUITY UPLC, Oasis, The Science of What's Possible, Xevo are trademark of Waters Corporation.
Waters Corporation
34 Maple Street, Milford, Massachusetts 01757, USA
tel. +1 508 478 2000 fax +1 508 478 1990
Website: www.waters.com

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.















