Direct Determination of 2-Ethylhexanoic Acid in Clavulanate
Dionex Application Note
Clavulanic acid is commonly used in combination with penicillin and cephalosporin antibiotics to overcome bacterial resistance. Clavulanic acid is produced and isolated from Streptomyces clavuligeru with ethyl acetate and then converted to a stable form. One secondary purification approach uses nonaqueous precipitation of potassium clavulanate by adding the potassium salt of 2-ethylhexanoic acid in isopropanol to the primary isolation extract. While 2-ethylhexanoic acid is soluble in the organic solvent, a small fraction may co-precipitate with the potassium clavulanate.
The United States Pharmacopeia (USP) monograph for potassium clavulanate describes a GC–FID method to determine 2-ethylhexanoic acid in the active pharmaceutical ingredient (API). This contribution describes a simpler approach for determining 2-ethylhexanoic acid in clavulanate with a Reagent-Free Ion Chromatography (RFIC) system. USP clavulanate reference standards were prepared at a 150-fold lower concentration in deionized water than the current USP method, spiked with 2-ethylhexanoic acid, and injected directly without additional sample pretreatment. The target analyte was separated from clavulanate-related peaks using electrolytically generated potassium hydroxide eluent and measured by suppressed conductivity detection.
Experimental
Analyses are performed using a Dionex ICS-2100, AS Autosampler and Chromeleon Chromatography Workstation. Separations use an IonPac AG11/AS11 column set (USP L61) with electrolytically generated KOH eluent.1
Results and Discussion
Figure 1 compares 500 µg/mL clavulanate with and without 2-ethylhexanoic acid spiked at the 0.8% acceptance criterion cutoff value specified in the USP monograph. As shown, 2-ethylhexanoic acid is separated in less than 10 min without any interference from minor components in the sample matrix. The method demonstrated good sensitivity with a low limit of detection (LOD) for 2-ethylhexanoic acid of 0.036 µg/mL, which corresponds to a concentration of 0.008% in the API. This represents a 100-fold lower concentration than the 0.8% acceptance criterion cutoff value in clavulanate. Results for linearity, spike recovery, retention time precision and peak area precision determinations show that IC is an accurate and reproducible technique to determine 2-ethylhexanoic acid in clavulanate below the 0.8% acceptance criterion. The lower LOD achieved by this method allows a 150-fold lower API concentration, thereby reducing cost. In addition, this proposed method eliminates the need for two solvent extractions required in the GC–FID method in the current USP monograph, thus significantly simplifying sample preparation, reducing waste, and further improving cost efficiency.
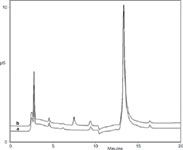
Figure 1: Chromatographic comparison of clavulanate without [trace (a)] and with [trace (b)] 4 µg/mL (0.8%) 2-ethylhexanoic acid. Peak 1: 2-ethylhexanoic acid (4 µg/mL â 0.8%). Eluent: 3 mM KOH from 0â10 min; 3â60 mM KOH from 10â10.1 min; 60 mM KOH from 10.1â20.1 min. Flow-rate: 0.25 mL/min. Temp.: 30 °C. Inj. Vol.: 5 µL. Detection: suppressed conductivity, ASRS 300 2 mm, recycle mode,2 mA suppressor current during 3 mM KOH, switch to 38 mA at 10.1 min.
Reference
1. Dionex Corporation. Determination of 2-Ethylhexanoic Acid Impurity in Clavulanate Using a Reagent-Free Ion Chromatography System. Application Note 262 (LPN 2608, Oct., 2010); Sunnyvale, California, USA
Chromeleon, IonPac, and RFIC are registered trademarks of Dionex Corporation.
Dionex Corporation
1228 Titan Way, PO Box 3603, Sunnyvale, California 940883603, USA
tel. (408)737-0700 fax (408)730-9403
Website: www.dionex.com

Biopharmaceutical Characterization in the Age of Artificial Intelligence
May 13th 2025AI-powered tools are enhancing precision, efficiency, and decision-making in biopharmaceutical development. Recently, Jared Auclair and Anurag Rathore explored AI's evolving role in biopharmaceuticals in detail.
Determining Ways to Protect Honeybee Colonies with GC–MS
May 13th 2025A study conducted by the Agriculture Research Centre of Giza, Egypt, and Jilin Agricultural University in China, evaluated the efficacy of stinging nettle extract, nettle smoke, and formic acid in the controlling of Varroa mites, a major threat to honeybee colonies, with a focus on mite infestation reduction, honeybee mortality, and biochemical responses. Gas chromatography–mass spectrometry (GC–MS) was used to identify key bioactive compounds in the stinging nettle extract.
Evaluating the Accuracy of Mass Spectrometry Spectral Databases
May 12th 2025Mass spectrometry (MS) can be effective in identifying unknown compounds, though this can be complicated if spectra is outside of known databases. Researchers aimed to test MS databases using electron–ionization (EI)–MS.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














