Free Simulators for Virtual Chromatography Laboratory Experiments
When forced to teach separation science with little or no access to a laboratory, chromatography simulators can help. Here, we describe several that are both useful and free.
When forced to teach separation science with little or no access to a laboratory, using chromatography simulators can help. Here, we describe several that are free. Regular readers of LCGC will be familiar with the powerful chromatography method development software advertised in this publication. Such software facilitates development and optimization of liquid chromatography (LC) and gas chromatography (GC) methods for novel compounds and separations. Virtual laboratory experiments using such software have been described (1,2). The simulators discussed below are limited to analytes and compounds within their embedded data, but are nonetheless useful in educational settings.
“ProEZGC” from Restek Corporation is a gas chromatography simulator to aid column selection and method development (3,4). Simulations are based on a database of thermodynamic data for nearly 8000 compounds collected on more than 30 GC stationary phases. Only compounds in the database can be modeled. When provided a list of compounds, ProEZGC suggests columns and simulates the chromatogram under optimum conditions. The effects of column length (L), internal diameter (dcol), film thickness (df), carrier gas, flow rate (F), or linear velocity, temperature program (up to five ramps) and detector pressure (vacuum or non-vacuum) can be modeled. Ideal injection and Gaussian peaks are assumed. Mass spectra are available for many compounds. Instructors and students must register for free access to the program. A video introduction to ProEZGC is available (5).
“HPLC Teaching Assistant” (6,7) is an Excel-based reversed-phase LC simulator with sheets focused on efficiency (L, dcol, and particle size [dp]), resolution (k, α, N), isocratic (% methanol) and gradient elution (initial and final % methanol, gradient time [tG] F, L, dcol, and dp), extracolumn broadening, and analyte characteristics (log P, pKa, and molecular weight). A widely-used textbook provides exercises using this simulator to illustrate fundamental chromatographic behavior and reversed-phase behavior (8).
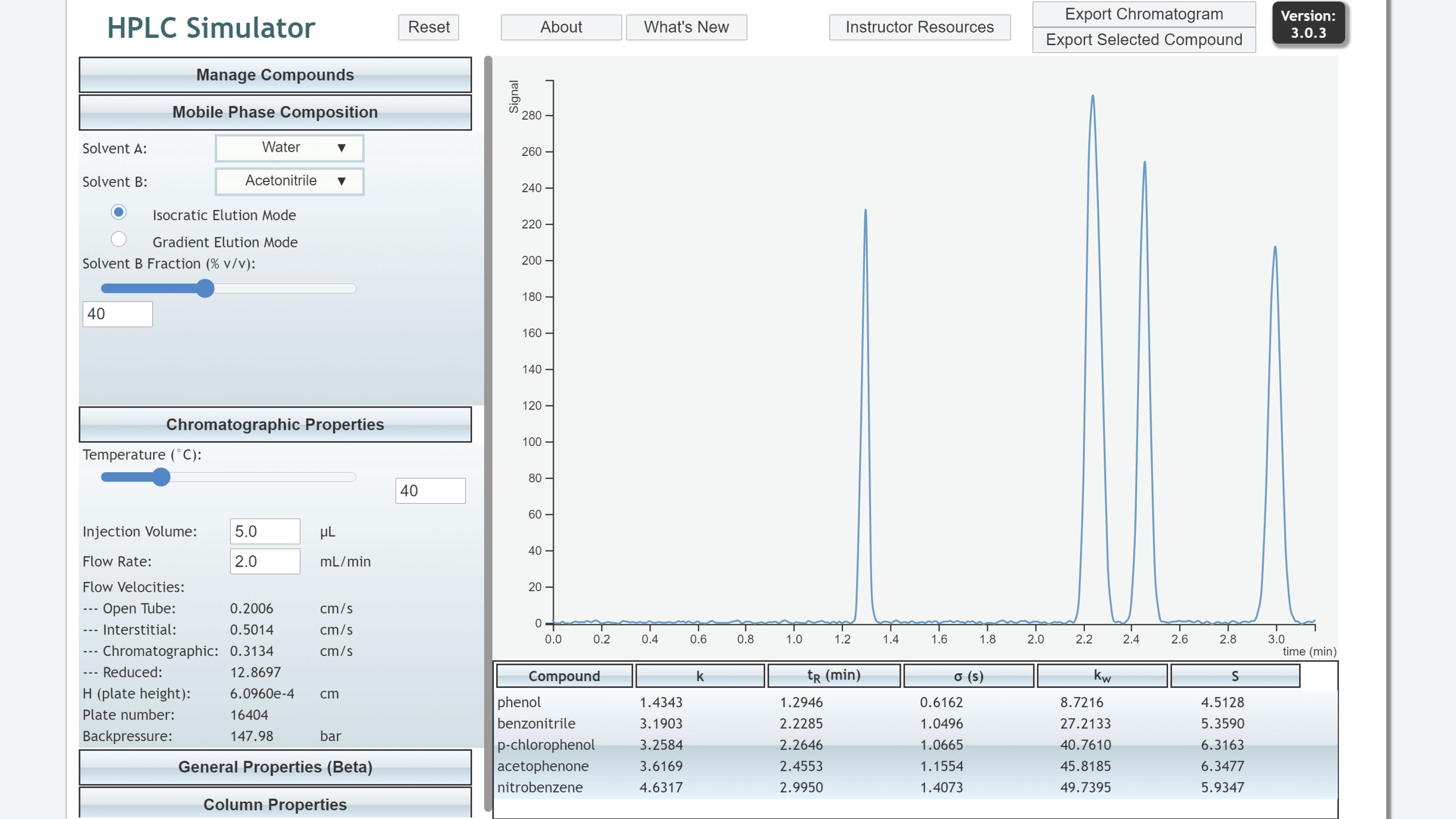
“HPLC Simulator” is a high fidelity simulation of reversed-phase high-performance liquid chromatography (HPLC) (9,10). Retention behavior for more than 40 compounds on an Agilent SB-C18 column is simulated using experimentally determined linear solvent strength parameters for methanol and acetonitrile. As shown in Figure 1, user-controlled parameters include organic modifier, %B, column temperature [T], dp, L, F, injection volume, and baseline offset and noise. Outputs for isocratic separations include the chromatogram (exportable to csv file), flow velocity, plate height (H), back pressure, a table of k, tR, peak width (σ), and the linear solvent strength parameters kw and S. Gradient inputs include gradient delay volume and outputs include retention factor at elution (ke). Virtual exercises and laboratory procedures are available through the Instructor Resources button for course levels ranging from sophomore to graduate. The site also has an excellent compilation of HPLC resources (11).
Figure 1: HPLC Simulator for reversed-phase LC separations.
“AppletChrom” animates the separation of five components within a column based on a Craig counter-current model (12). Plate number, distribution ratios, and detector response factors can be varied. This, and 23 other applets relevant to analytical chemistry, were recently rewritten in JavaScript and can run on most common browsers.
“Virtual Column Online” from Thermo Fisher Scientific is an ion chromatography (IC) simulator to aid column selection and method development (13,14). Experimentally determined parameters are used to simulate separations for: 89 inorganic and organic anions on 15 anion exchange columns for isocratic carbonate or bicarbonate, isocratic hydroxide, and linear-gradient hydroxide; 111 inorganic and organic cations on 5 cation exchange columns using isocratic and linear gradient methanesulfonic acid; and 32 carbohydrates on a CarboPac PA20 column using isocratic and linear-gradient hydroxide. Up to three column temperatures can be modeled. Predicted peak efficiencies and asymmetry reflect typical column performance, but do not exhibit van Deemter behavior. Users must sign up for the AppsLab Library for free access to the simulator. A 25-page getting-started guide is available in English, Japanese, and Chinese. A procedure for a virtual IC laboratory is being prepared by the author.
“PeakMaster” simulates capillary electrophoresis including electromigration dispersion, indirect detection, and system peaks (15–17). Users input capillary length, voltage, and electro-osmotic mobility or neutral marker time, and select background electrolyte components and analytes from comprehensive pull-down menus. Clicking “calculate” yields a simulated electropherogram showing analyte peaks and system peaks.
Many instructors will be facing this fall with trepidation given the loss or restrictions on tried-and-true laboratory instruction. Fortunately, there are many tools available to simulate separation techniques. These simulators allow students to explore the instrument behavior with much more freedom than possible in traditional laboratories. Furthermore, all students can work on a laboratory at the same time, allowing the instructor to integrate the lecture and simulation experiments. So, look at this fall as an opportunity to explore what these simulators have to offer. All the best to you and your students.
References
- D.C. Stone, J. Chem. Ed. 84, 1488–1496 (2007).
- https://sites.chem.utoronto.ca/chemistry/dstone/Research/virtual.html
- www.restek.com/ezgc
- J. de Zeeuw, The Analytical Scientist, Issue 71, 38–41 (December 2018).
- www.restek.com/Technical-Resources/Technical-Library/Video-Library/Pro-EZGC-Chromatogram-Modeler-Introduction
- D. Guillarme and J.L. Veuthey, LCGC North Am. 34(10), 804–811 (2016) and 34(12), 906–915 (2016).
- https://ispso.unige.ch/labs/fanal/hplc_teaching:en
- D.C. Harris and C.A. Lucy, Quantitative Chemical Analysis (W.H. Freeman and Co., New York, New York, 10th ed., 2020), problems 23-30, 23-55, 23-56, 25-48, 25-50.
- P.G. Boswell, D.R. Stoll, P.W. Carr, M.L. Nagel, M.F. Vitha, and G.A. Mabbott, J. Chem. Ed. 90, 198–202 (2013).
- www.multidlc.org/hplcsim/hplcsim.html
- http://www.multidlc.org/hplc_resources
- C.E. Efstathiou, University of Athens, http://195.134.76.37/applets/AppletChrom/Appl_Chrom2.html
- https://appslab.thermofisher.com/ Click on “Virtual Column Online” in the top right horizontal menu.
- P.R. Haddad, M.J. Shaw, J.E. Madden, and G.W. Dicinoski, J. Chem. Ed. 81, 1293–1298 (2004).
- (B. Gaš, M. Jaroš, V. Hruška, I. Zuskova, and M. Štědrý, LCGC Europe 18(5), 282–288 (2005).
- https://web.natur.cuni.cz/gas/peakmaster.html
- M. Jaroš, V. Hruška, M. Štědrý, I. Zusková, and B. Gaš, Electrophoresis 25, 3080–3085 (2004).
Charles A. Lucy is a professor emeritus in the Department of Chemistry at the Gunning/Lemieux Chemistry Centre at the University of Alberta in Edmonton, Canada. Direct correspondence to charles.lucy@ualberta.ca

The LCGC Blog: ACS SCSC: What We Do and Who We Are
November 30th 2021In this month’s blog, we provide information about the Subdivision on Chromatography and Separations Chemistry (SCSC) of the Analytical Division of the American Chemical Society (ACS), sharing our main goals and introducing our newly appointed executive board members.
The LCGC Blog: Inexpensive, Quick, and Selective: Seeking the Holy Grail of Sample Extraction
September 8th 2020Oliver Napoleon Hill (1883– 1970) was an American self-help author once described as ”the most famous conman you’ve probably never heard of” (1 ). Conman maybe, but there is a quote of his that I believe to be particularly true when considering sample preparation for chromatography techniques; ”The one who tries to get something for nothing generally winds up getting nothing for something.”
The LCGC Blog: Miniaturized Chromatography—The Next Big Idea
September 2nd 2020In any given community, on rare occasions, leaders converge on a big idea. The development of such an idea alters the course of the community, in a way that is meaningful if it is lasting. It is my belief that the collective chromatography community is currently converging on a big idea.







