Fraction Collection and Simultaneous Concentration for Chiral Purifications Using Supercritical Fluid Chromatography (SFC)
A novel centrifugal fraction collector CFCâ„¢ enables SFC users to simultaneously collect and concentrate fractions. The technique is particularly useful for chiral separations where large volumes of enantiomers are being purified by stacked injection methods.
Herbert J. Hedberg, Modular SFC, Inc.
A novel centrifugal fraction collector CFC™ enables SFC users to simultaneously collect and concentrate fractions. The technique is particularly useful for chiral separations where large volumes of enantiomers are being purified by stacked injection methods.
SFC has proven particularly useful in drug discovery for providing pure enantiomers in support of scale-up synthesis. In most cases collected materials must be dried and concentrated before moving to the next step. Productivity can be significantly increased if the two serial processes of collection and concentration could be combined to occur simultaneously.

Figure 1
Modular SFC has developed a novel, centrifugal fraction collector capable of collecting large volume fractions and simultaneously concentrating the fractions in the collection containers. Because the concentration process occurs simultaneously with the fractionation activity, the instrument significantly speeds the overall process from injection to dry pure compound.
Technology
Modular SFC's CFC-2™ Chiral Centrifugal Fraction Collection System operates at atmospheric pressure and can accommodate four 250 mL glass containers* to collect fractions.
A rotor containing the collection bottles spins at 1,500 rpm (concentrator speed). Flexible tubing is redirectable to each fraction while the rotor continues to spin. The centrifugal force causes the highest density components (liquids and solids) to accumulate in the bottom of the bottles while the expanded gaseous components spill into the confines of the rotor chamber.
The Centri-Fan (patent pending) recirculates room temperature or heated rotor chamber gas into each media bottle at 50 cfm while the rotor spins and fractions are being collected. The centrifugal force assures high fraction recovery despite the aggressive blow-down gas flow rate.
Results
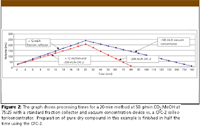
Figure 2
Conclusion
The CFC-2 Chiral Centrifugal Fraction Collector with simultaneous concentration capability combines typically serial process steps to speed overall process time. The centrifuge-like aspects of the instrument make it especially effective at recovering sample from SFC eluant flows; however, even HPLC-based methods will benefit from the productivity gains provided by simultaneous concentration.
*Various rotors for the CFC-2 Chiral Centrifugal Fraction Collector can accommodate 24 × 20 mm × 150 mm glass tubes; 4 × 250 mL media bottles; 4 or 8 × 100 mL round evaporator flasks; 4 or 8 × 20 mL or 40 mL scintilation vials.
References
(1) Berger T., Smith J., Fogelman, K., Kruluts K. American Clinical Laboratory 2002: October.
(2) Wu DR, "Chiral Supercritical Fluid Chromatography in Drug Discovery: from Analytical to Multigram-Scale," presented at the 2007 SFC Conference, August 31– September 3, 2007, Dallas, Texas.

Modular SFC, Inc.
167 S. Washington St.,
North Attleboro, MA 02760-2235
Tel: (508) 479-1161; Fax: (508) 695-1958
Email: info@modularsfc.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














