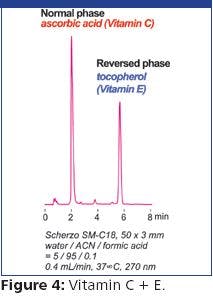
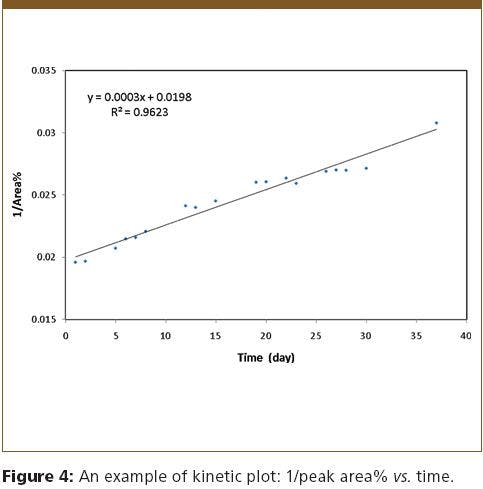
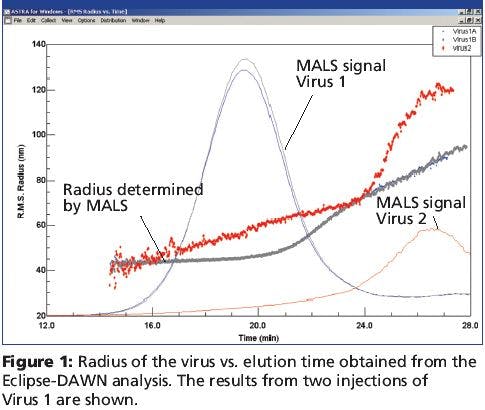
Flexible Fused Silica Capillary: A Discussion of Strength
Synthetic fused silica capillary tubing is a vital component in many scientific techniques. The general perception is that most laboratory glass products are fragile and easy to break. The opposite is true of fused silica capillary; with its protective coating it is both strong and durable when handled properly.
Synthetic fused silica capillary tubing is a vital component in many scientific techniques. The general perception is that most laboratory glass products are fragile and easy to break. The opposite is true of fused silica capillary; with its protective coating it is both strong and durable when handled properly.
Researchers routinely use synthetic fused silica capillary in performing GC, CE, capillary LC, cytometry, and related analytical analysis techniques. Regardless of the application, the fundamental question of capillary strength often arises. This note discusses fused silica capillary tensile strength, strength testing, and the key user activities that can impact strength.
Fused Silica Strength
Flexible synthetic fused silica capillary tubing is drawn from high purity, low metal ion content Silicon dioxide preforms. In most cases the fused silica tubing is externally coated with an abrasion resistant material such as polyimide; this coating protects the surface from external damage, resulting in a durable high-strength product. The theoretical tensile strength based on Si-O bond energy calculates to be ~2,000 kpsi. The empirical strength of fused silica has a lower value due to minor imperfections within the amorphous structure and at the surface; a typical value is ~700 kpsi.
Strength Testing and Screening
The most common method employed for determining capillary strength is a tensile test. This destructive test is performed on a Tensile Tester, such as an Instron Model 3344 (Instron, Norwood, Massachusetts). A section of tubing ~2 m in length is loaded into the device and then pulled at a defined strain rate, typically 0.25 m/min, until failure occurs. The load at failure is recorded for a sample set of 20, with the corresponding statistical data presented in the form of a Weibull Plot (1). Alternatively, a two-point bend test can be performed wherein the radius of the bend at failure is recorded and the corresponding tension is back calculated using Young's Modulus. It should be noted that the measured strength will decrease as the glass substrate increases in size; larger material is statistically more likely to contain a failure inducing imperfection.
Strength screening is of equal importance in capillary manufacturing. A bend radius test in no less than two axes is performed in-line during the draw process. The capillary is routed through a series of rollers; their diameter is selected to correspond to a desired stress level. The technique is called Proof Testing, with the typical applied stress being 100 kpsi. This approach allows for 100% screening, as the test is not destructive. For comparison, 0.53 mm i.d. capillary placed on a typical 8" GC cage will experience ~30 kpsi of stress.
A common misconception is that bend radius screening is related to internal pressure capability; no such relationship is suggested here. Pressure testing would be a separate evaluation; the topic has been discussed previously (2).
Common Factors Which Impact Strength
Anything that will cause a flaw to form in the fused silica should be considered. The most common factor is improper handling, wherein the protective coating is inadvertently penetrated and the underlying fused silica surface compromised. Introduction of debris into the capillary may damage the internal surfaces, which will immediately compromise strength. Common causes are poor cleaving technique, use of unfiltered reagents, and dirty components upstream in the flow path, including residue from cleaving (3). Exposure to atmospheric gases, including water, over time will result in an aging effect by accelerating the propagation of surface imperfections. This is why the capillary is sealed with an inert gas inside upon manufacture (4).
Conclusion
Polyimide coated fused silica capillary is a very strong material. It is continuously monitored for strength during its manufacture by proof testing. With proper handling and usage, it will retain excellent strength in most applications. For specific questions, contact a Polymicro technical specialist.
References
(1) The Book on the Technologies of Polymicro, 2005, p 2.17.
(2) J. Macomber, C.R.Forest, LCGC Application Notebook, Sept 2006, p.2.
(3) J.Macomber, L.Begay, LCGC Application Notebook, Sept 2003, p.72.
(4) J.Macomber, D.Stasiak, LCGC Application Notebook, June 2008, p.63.

Polymicro Technologies
A subsidiary of Molex Incorporated
18019 N 25th Ave., Phoenix, AZ 85023-1200
tel. (602)375-4100, fax (602)375-4110
Website: www.polymicro.com

Automated Sample Preparation (ISO 20122) for MOSH/MOAH in Seasoning Oils
May 6th 2025This work presents an Automated Sample Preparation procedure for MOSH/MOAH analysis of Seasoning Oils. We compare results from a manual epoxidation procedure compliant with DIN 16995 with results based on fully automated sample preparation (epoxidation and saponification) compliant with ISO 20122. In both cases, online clean-up via activated aluminum oxide (AlOx) are used to remove interfering n-alkanes from the MOSH fraction during the HPLC run. Automated data evaluation using a dedicated software (GERSTEL ChroMOH) is presented.
Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)