Profiling the Isomerization of Biologically Relevant (E)-(Z) Isomers by Supercritical Fluid Chromatography (SFC)
In the past few decades, single enantiomers and stereoisomers have overtaken achiral molecules in the percentage of approved drugs in the market. Because isomers can have different biological/pharmacological/toxicological properties, authorities, such as the European Pharmacopoeia and the FDA, have asserted escalated emphasis on controlling isomer content in drug compounds and require that stereoisomers, "be treated as separate drugs and developed accordingly" with rare exception. The separation and quantification of stereoisomers is therefore of great importance, especially when considering pharmaceutical compounds (1).
Jacquelyn Cole(1), Rui Chen(1), Billy W. Day(2), and Vasiliy N. Korotochenko(2) (1)TharSFC, a Waters Company and (2)Department of Pharmaceutical Sciences, University of Pittsburgh
In the past few decades, single enantiomers and stereoisomers have overtaken achiral molecules in the percentage of approved drugs in the market. Because isomers can have different biological/pharmacological/toxicological properties, authorities, such as the European Pharmacopoeia and the FDA, have asserted escalated emphasis on controlling isomer content in drug compounds and require that stereoisomers, "be treated as separate drugs and developed accordingly" with rare exception. The separation and quantification of stereoisomers is therefore of great importance, especially when considering pharmaceutical compounds (1).
Supercritical fluid chromatography (SFC) has become the choice of chromatography for separating stereoisomers owing to its speed, efficiency, and cost-effectiveness (2). However, SFC has been primarily used to separate optical isomers, including enantiomers and diastereoisomers. Its applications on (E)-(Z) isomers are limited in scope (3).
We present herein a case study employing SFC to profile the (E)-(Z) transformation of (E)-2-benzylidene-3-(cyclohexylamino)-2,3-dihydro-1H-inden-1-one (BCI), a small molecule inhibitor of dual specificity phosphatase 6 (Dusp6). BCI is a synthetic molecule which contains an asymmetrical carbon and a sp2-hybridized C-C double bond resulting in both optical and (E)-(Z) isomerism. A six-min SFC method was developed to simultaneously separate all four isomers. The method was subsequently employed to profile the (E)-(Z) isomerization of the BCI molecule over the course of 40 days.
Experimental
Materials: The (E)-2-benzylidene-3-(cyclohexylamino)-2,3-dihydro-1H-inden-1-one (BCI) was a gift from Professor Billy Day at the Department of Pharmaceutical Science and Chemistry, University of Pittsburgh. The chemical structures of the BCI (E)-(Z) isomers are shown in Figure 1. BCI was dissolved in HPLC grade methanol with an estimated concentration of 1 mg/ml for method development. A diluted sample (0.05 mg/ml) was used to profile the isomerization. The chiral stationary phases used in this study were Chiralpak AD-H, Chiralpak AS-H, Chiralcel OD-H, and Chiralcel OJ-H. All columns were 4.6 × 250 mm in dimension.
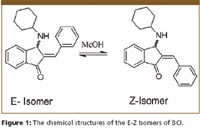
Figure 1
Chromatography: All experiments were carried out using a TharSFC Investigator (TharSFC, a Waters Company, Pittsburgh, Pennsylvania). The system was equipped with a Waters 2998 PDA detector (Milford, Massachusetts). For all experiments, the key experimental parameters were as follows: the flow rate was 4 ml/min, the back pressure was 100 bar, the temperature was 40 °C, the PDA scan was 220–300 nm, and the injection volume was 10 μL. The initial screening was done using a linear gradient: 2% to 25% methanol in 6 min, holding at 25% for 4 min, and returning to 2% in 1 min. The optimal method for isomerization study was: 5% to 25% in 5 min, holding at 25% for 3 min and returning to 5% in 1 min.
Results and Discussion
Figure 2 shows the method screening for the chiral separation of the E-isomer. Both AD-H and OJ-H columns yielded baseline resolution, whereas AS-H and OD-H offered no resolution of the enantiomeric pair of the E-isomer. OJ-H was selected for the ensuing isomerization study due to the shorter analysis time.

Figure 2
At day 5, a pair of peaks, immediately adjacent to the enantiomeric pair of the E-isomer, started to emerge (Figure 3). The peaks were collected, analyzed by NMR (results not shown), and confirmed to be the enantiomers of the Z-isomer. Over the course of 40 days, a gradual transformation from E- to Z-isomer was observed. Representative chromatograms are shown in Figure 3.
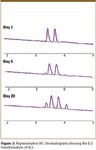
Figure 3
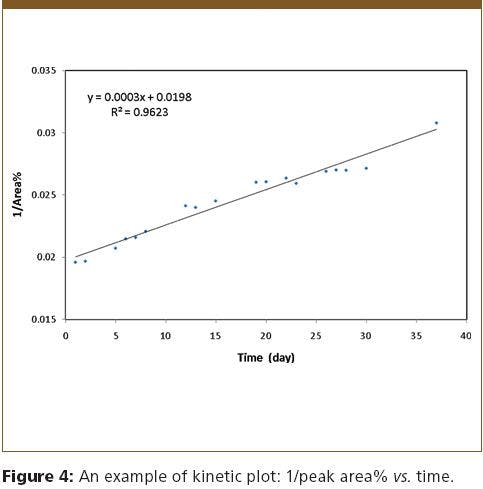
Since both E- and Z- isomers are racemic, the peak area% of one E-isomer and its derivatives was plotted against time to gauge the rate of the observed isomerization. Shown in Figure 4 is the plot of 1/peak area% vs. time, with a R2 of 0.9623, suggesting second-order reaction kinetics. It is noted such data maneuvers can be misleading and more careful experiments are required to fully understand the reaction kinetics. Nevertheless, simultaneous baseline resolution of all four isomers by SFC is undoubtedly an essential part of such a study.

Figure 4
Conclusions
In this report, we have demonstrated the simultaneous resolution of all four isomers of BCI, both chiral and (E)-(Z) isomeric, by SFC in less than 5 min. The developed SFC method also holds potential for the kinetic study of (E)-(Z) isomerization.
References
(1) H. Caner, E. Groner and L. Levy, Drug Discovery Today, 9(3), 105–110 (2004).
(2) D. Mangelings and Y. V. Heyden J. Sep. Sci. 31, 1252–1273 (2008).
(3) F. Hasdenteufel, Separation & Purification Reviews, 35, 193–221 (2006).
Acknowledgement
JC and RC are grateful for the generous gift of BCI from Professor Billy Day at the Department of Pharmaceutical Science and Chemistry, University of Pittsburgh.
BWD and VNK would like to thank the NIH (grant number HL088016) for the financial support.

TharSFC, a Waters Company
575 Epsilon Drive, Suite 100, Pittsburgh, PA 15238
tel. (412)967-5665, fax (412)967-9446
Email: info@tharsfc.com Website: www.waters.com/sfc

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)