Fast Separation of Eleven Nitroaromatic Compounds on ZirChrom-CARB
This technical bulletin details the separation of 11 closely related nitroaromatic compounds, namely RDX, HMX, Nitrobenzene, 2-Nitrotoluene, Tetryl, 2,6-Dinitrotoluene, 4-Nitrotoluene, 1,3-Dinitrobenzene, 2,4-Dinitrotoluene, 2-amino 4,6-dinitrotoluene, 1,3,5-Trinitrobenzene. Our customers have reported that similar separations on silica-based phases produce run times as long as 30 min. Here we report a method on ZirChrom?-CARB at a column temperature of 125 ?C in under 4 min.
This technical bulletin details the separation of 11 closely related nitroaromatic compounds, namely RDX, HMX, Nitrobenzene, 2-Nitrotoluene, Tetryl, 2,6-Dinitrotoluene, 4-Nitrotoluene, 1,3-Dinitrobenzene, 2,4-Dinitrotoluene, 2-amino 4,6-dinitrotoluene, 1,3,5-Trinitrobenzene. Our customers have reported that similar separations on silica-based phases produce run times as long as 30 min. Here we report a method on ZirChrom® -CARB at a column temperature of 125 °C in under 4 min.
The rapid and accurate detection of nitroaromatic compounds is difficult due to the structurally similarity of the compounds. The unique surface chemistry of ZirChrom®-CARB enables reversed phase, ion exchange and pi-pi bond interactions. This multi-modal selectivity makes the ZirChrom®-CARB phase an excellent choice for the analysis of structurally similar compounds.
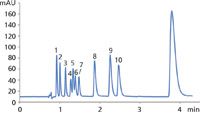
Figure 1: Separation of 11 nitroaromatics on ZirChrom®-CARB. 1 = RDX, 2 = HMX, 3 = Nitrobenzene, 4 = 2-Nitrotoluene, 5 = Tetryl, 6 = 2,6-Dinitrotoluene, 7 = 4-Nitrotoluene, 8 = 1,3-Dinitrobenzene, 9 = 2,4-Dinitrotoluene, 10 = 2-amino 4,6-dinitrotoluene, 11 = 1,3,5-Trinitrobenzene.
The ZirChrom®-CARB phase is stable up to temperatures of 200 °C. Performing separations at elevated temperatures reduces mobile phase viscosity and improves mass transfer. The resulting decrease in backpressure allows for faster flow rates and translates into significantly shorter run times without the loss of sample resolution.
Experimental
A mixture of nitroaromatics was separated at 125 °C using a ZirChrom®-CARB column (aee Figure 1). The separation conditions were as follows:
Column: ZirChrom®-CARB, 150 mm × 4.6 mm i.d., (part # ZR01-1546)
Mobile phase: Isocratic Pre-mixed 35/15/50 Acetonitrile/Tetrahydrofuran/20 mM ammonium carbonate pH 5.7, 10 mM octylamine
Flow Rate: 2.0 mL/min
Temperature: 125 °C
Detection: 254 nm
Inj. Volume: 1 µL
This separation allows for clear identification of these compounds without the use of expensive MS detection. The separation is also completed using isocratic conditions, thus facilitating a more reproducible transfer from LC to LC.
Want to learn more about the unique selectivity and stability of ZirChrom phases? ZirChrom is pleased to announce our new, free, on-demand webinar series: ChromU. Webinar topics include the use of elevated temperature and ZirChrom carbon phases. ChromU webinars are, on average, less than 10 min in length and include links to helpful supplemental application notes and newsletters. ChromU is available 24 hours a day, seven days a week on the ZirChrom website www.zirchrom.com. Find your ZirChrom solution today!
ZirChrom Separations, Inc.
617 Pierce Street, Anoka, MN 55303
tel.1-866-STABLE-1
Email: support@zirchrom.com, Website: www.zirchrom.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














