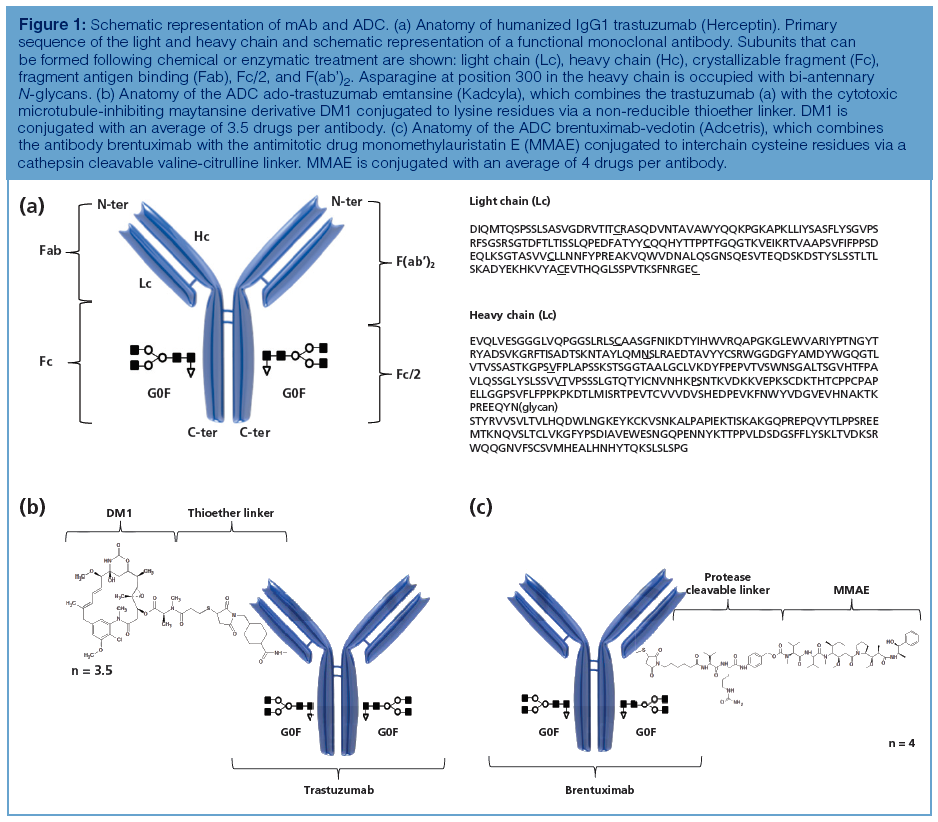
Extending the Hydrocarbon Range for the Analysis of Soil Gas Samples Using Automated Thermal Desorption Coupled with Gas Chromatography–Mass Spectrometry
LCGC Europe
This study describes the recovery of compounds above the boiling point of naphthalene achieved by optimizing the thermal desorption chemistry for the determination of volatile organic compounds ranging from C3 to C26 in soil gas samples using Method TO-17. Figures of merit such as breakthrough, precision, linearity, and detection capability are presented, in addition to an evaluation of its real-world capability at sites with moderate diesel and semivolatile polynuclear aromatic hydrocarbon (up to pyrene) contamination, in the presence of high humidity. This research has provided a means to determine a more representative composition of soil gas.
Photo Credit: taffpixture/Shutterstock.com

Lee Marotta1, Stephen Varisco2, Miles Snow1, Tom Kwoka1, and Robert Thomas3, 1PerkinElmer Inc., Shelton, Connecticut, USA, 2CARO Analytical Services, Richmond, British Columbia, Canada, 3Scientific Solutions, Gaithersburg, Maryland, USA
This study describes the recovery of compounds above the boiling point of naphthalene achieved by optimizing the thermal desorption chemistry for the determination of volatile organic compounds ranging from C3 to C26 in soil gas samples using Method TO-17. Figures of merit such as breakthrough, precision, linearity, and detection capability are presented, in addition to an evaluation of its real-world capability at sites with moderate diesel and semivolatile polynuclear aromatic hydrocarbon (up to pyrene) contamination, in the presence of high humidity. This research has provided a means to determine a more representative composition of soil gas.
In 1994 the United States Environmental Protection Agency (US EPA) published a report that an average person breathes approximately 20,000 litres of air per day (1). Since then, the need to optimize methods for the analysis of toxic compounds in air to understand their impact to human health has increased significantly. The scientific and regulatory communities have long been aware of the potential for migration of vapours from contaminated groundwater or soil into buildings, but until recently (with the exception of radon and major fuel leaks) soil vapour intrusion has not been a major concern. Then in the late-1990s, two sites in Colorado with chlorinated solvent plumes were found to have contributed to the contamination of a number of residential buildings. In 2002, the EPA issued draft guidance that provided technical and policy recommendations for determining if the vapour intrusion pathway posed a risk to human health at cleanup sites. Since then, the majority of American states (2) and several Canadian provinces (3) have introduced vapour intrusion guidelines and legislation.
Current EPA methods for volatile organic compounds (VOCs) in air are Method TO-15 using a Summa canister (4) and Method TO-17 using a sorbent tube (5). However, the target analytes specify a boiling point range of only C3 to C12. For that reason, efficient methods are needed for the analysis of soil gases for toxic compounds above this range. For example, diesel fuel components are of great concern because they can be found in soil gas and are known to have an impact on human health. Additionally, the sampling and analysis of soil gas samples poses several unique challenges when compared to indoor or ambient air monitoring. For instance, soil gas often has a higher moisture content and possibly a broader range of compounds. Because the compounds are typically confined, some sites can be very contaminated. Thus, sorbent tubes and the analytical systems used need to deal with this while providing accurate data at the low detection limits required for the toxic regulated compounds.
It’s also important to emphasize the difference between the sampling procedures of these two EPA methods to fully understand these complex issues. Method TO-15 uses a large stainless steel vessel called a Summa canister, which collects approximately 6 L of air. A fraction of this sample volume, typically 500 mL, is withdrawn from the canister and sent to a concentrator sorbent trap. The sample is then desorbed from the trap and focused onto a gas chromatographic (GC) analytical column to be separated and analyzed by mass spectrometry (MS).
There are several limitations of the TO-15 approach. This method only reliably recovers up to naphthalene (C12) while several regulatory directives require measurements up to at least C13. In addition, many air samples might contain higher boiling substances that can adsorb onto the sides of the canister and condense. Other challenges include analyzing air samples with high moisture content and the requirement for a greater number of analytes (in some cases up to C40) over a wide range of concentrations. Method TO-17 overcomes many of these challenges by using a thermal desorption tube instead of a Summa canister to collect the sample. The thermal desorption process utilizes a sorbent tube, which contains adsorbent material specifically selected to trap the range of analytes of interest. In active sampling, a known volume of air is sampled through the tube, where the contents are then desorbed onto a secondary trap into the analytical column to be analyzed by GC–MS (6).
This study therefore describes the need to recover compounds above the boiling point of naphthalene by optimizing the thermal desorption chemistry for the determination of VOCs from C3 to C26 in soil gas samples using Method TO-17. Figures of merit such as breakthrough, precision, linearity, and detection capability are presented, in addition to evaluating its real-world capability at sites with moderate diesel and semivolatile polynuclear aromatic hydrocarbon (up to pyrene) contamination, in the presence of high humidity. Because compounds with boiling points higher than naphthalene were added to the analyte list, experiments were also performed to ensure that lighter compounds, such as vinyl chloride, remained on the tube during sampling. This research has provided a means to determine a more representative composition of soil gas.
Study Objectives
The selection of the tube material and the optimization of the thermal desorption chemistry were based on the following objectives:
- Extend the analyte range past naphthalene (the limit of typical sorbent tubes)
- Many contaminated sites have diesel contamination. The sorbent tubes need to adsorb these compounds separately from (that is, before) the more volatile ones during sampling
- Achieve good recovery for the higher boiling components during desorption.
- Ensure the most volatile compounds such as vinyl chloride do not break through the sorbents during sampling
- Ensure the sorbents selected did not produce target artifacts that may result in false positives
- Enable “quick clean up” of the tubes so that the primary desorption process would make them available for resampling, reducing analytical cost
- Maintain good water management inherent with using hydrophobic sorbents
- Increase sampling volumes to attain low reporting limits while enabling the recollection of the sample in case reinjection of the same sample is required.
Before we describe the experimental details of this study, let’s first describe the basic principles of processing samples using thermal desorption.
Fundamental Principles of Automated Thermal Desorption for Air Monitoring
After samples have been collected (or standards injected) onto the sorbent tubes, they are loaded onto the automated thermal desorption autosampler. The instrument inserts the tube into the primary desorption path. A leak check is performed on both the sample tube and the concentrator trap to ensure sample integrity. Additionally, an impedance check may be performed on the tube at this time to validate that the tube is packed properly (that is, there are no preferential pathways or the sorbents are not packed too tightly).
After these steps are performed, an inert gas flows through the tube, automatically introducing a gaseous internal standard onto the tube (this step is optional) while performing a dry purge to rid the tube of oxygen and water. Following the dry purge, a heater is placed onto the tube. Using a combination of heat, flow, and time, the contents of the tube are transferred to the concentrator (cold) trap, which is represented in Figure 1.
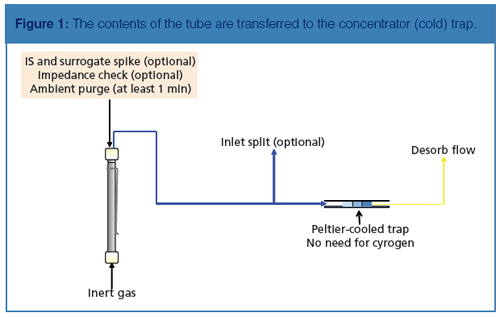
The concentrator trap uses a Peltier (electronic) cooler instead of the traditional liquid cryogen to achieve the trap’s lowest temperature of -35 °C if required. The low dead volume trap contains the same hydrophobic sorbents as the soil vapour intrusion tube; therefore, the analytes are focused in such a way that detectable breakthrough is prevented. After the contents of the tube are adsorbed onto the concentrator trap, it is heated rapidly and the contents of the trap are introduced into the GC analytical column in a narrow band, as shown in Figure 2.
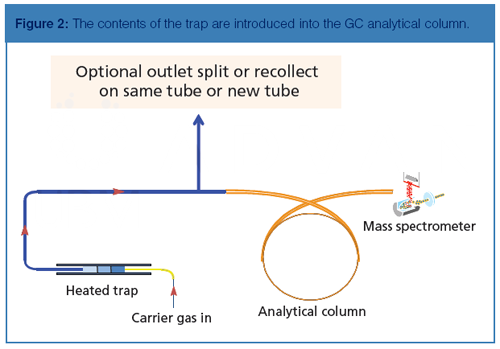
For highly concentrated samples, two splitters may be used: an inlet split between the sample tube and the concentrator trap, and an outlet split between the concentrator trap and the analytical column, enabling split ratios of several orders of magnitude. The inlet split may be disabled, and the outlet split may be used to recollect the sample onto the same tube or onto a different tube, preserving the sample for another injection (such as a sample dilution). For trace-level samples, splitless injection may be performed. Additionally, surrogates can be automatically spiked onto all tubes before sampling to provide additional data quality assurance.
Experimental
The instruments used in this application were a TurboMatrix Thermal Desorber 650, a Clarus 680 gas chromatograph, and a Clarus SQ8 mass spectrometer (both from PerkinElmer Inc.). Details of analytical parameters are described in Table 1.
A 60 m × 0.25 mm, 1.4-µm df Restek–624Sil MS column was chosen for this work because it allows a maximum temperature of 320 °C, which is required to elute the polynuclear aromatic hydrocarbons (PAHs) that are heavier than naphthalene. The mass spectrometer was operated in full scan mode, achieving the necessary detection limit criterion without the need for selective ion monitoring (SIM).
A total of 86 target compounds and diesel was investigated. Diesel was purchased from a nearby gas station. The following standards were purchased from Restek Corporation:
- 502.2 calibration mix 1 containing six gases
- 1,3-Butadiene
- 8260B Mega Mix containing 76 VOCs
- 2-Methylnaphthalene, anthracene, fluorene, and phenanthrene from separate ampules
The standards were diluted with purge-and-trap-grade methanol to attain the required concentrations for the following experiments.
Breakthrough and Recovery: The breakthrough volume is a critical factor in the performance of an adsorbent. It is defined according to EPA TO-17 as the volume sampled when the amount of analyte collected in a backup sorbent tube reaches 5% of the total amount collected by both sorbent tubes. Another very important performance criterion is spike recovery, which involves the analysis of a spiked tube from the breakthrough test, by first analyzing a blank tube and then reanalyzing the spiked tube to see if all analytes were desorbed off the tube from the first desorption. This analysis was performed by spiking the thermal desorption tubes with a high concentration of the following analytes to mimic a contaminated site:
- 300 ng of the 502.2 calibration mix (six gases)
- 300 ng of 1,3-butadiene
- 300 ng of 8260B Mega Mix
- 300 ng of the four PAHs
- 10 µg of diesel
After spiking three tubes, each tube was connected to a clean tube referred to as the breakthrough check tube. Each set (spiked tube connected to breakthrough check tube) was placed on a manifold that accommodated three sets. Then 100 mL/min of humidified nitrogen (85%) was simultaneously passed through the tubes for 100 min to simulate a 10-L sampling volume. This process is exemplified in Figure 3, which provides an illustration of the experiment.
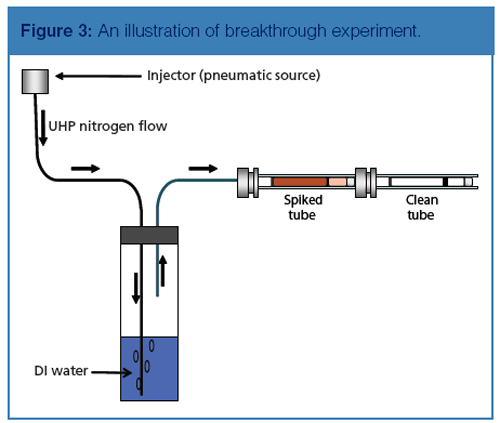
The goal was that none of the target compounds would be detectable in the breakthrough check tube (the second tube in the series). An assessment of the recovery of the desorption process was confirmed by reanalyzing the spiked tube. A trap test was performed first to ensure the targets were recovered from the trap. Then an empty (blank) tube was analyzed before the reanalysis of the spiked tube to confirm instrument cleanliness.
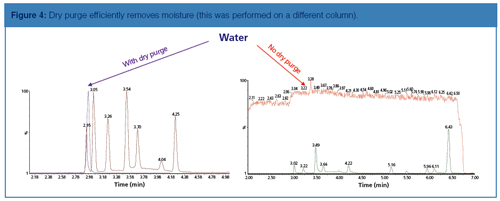
Water Management: The retention of water was determined by starting the MS scan at 15 amu instead of 35; therefore, enabling the mass characteristic of water at 18 amu to be acquired. Figure 4 illustrates the level of water management achieved by using this dry purge system, essentially reducing moisture content to instrument background levels.
Instrument Precision: Instrument precision was investigated by spiking 10 tubes with a 20-µg/mL VOC standard (4 ng on column).
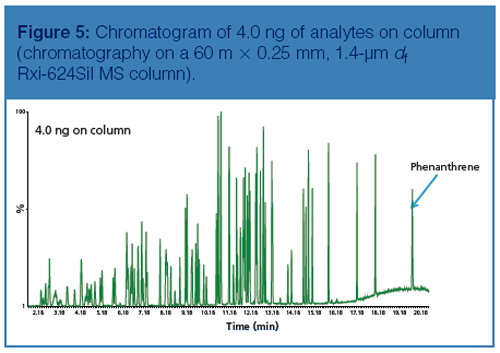
Chromatography: Figure 5 represents a total ion chromatogram (TIC) of a standard representing 4.0 ng on column.
Method Dynamic Range: The method dynamic range was evaluated over four orders of magnitude, from 0.05–250 ng spiked onto the tube. For a 1-L sample, this equates to a concentration range of 0.05–250 µg/m3; therefore, with a 1-L sample volume, a reporting limit of 0.05 µg/m3 is achieved across the compound target list. However, several targets can achieve a lower reporting limit.
Detection Capability: The detection capability of the method was also investigated as outlined in the summary of results below.
Internal Standard Precision: Internal standard precision was also investigated. This process is automated by the thermal desorber; 15 tubes were inserted onto the instrument’s carousel and spiked with an internal standard.
Tuning Criterion: The tuning criterion was met for TO-17 using 4-bromofluorobenzene (BFB).
Summary of Results
With 10 L of humidified nitrogen flowing through the tubes, the results of the breakthrough experiment were minimal, with only one of the most volatile compounds, dichlorodifluoromethane (Freon-12), observed at less than 1%. Vinyl chloride, one of the most toxic volatile gases, exhibited no detectable breakthrough, despite the fact that the concentration and humidity of the tubes were very high. Retaining this compound is critical since its toxicology is well documented. The full set recovery data for a group of polynuclear aromatic hydrocarbon compounds is shown in Table 2.
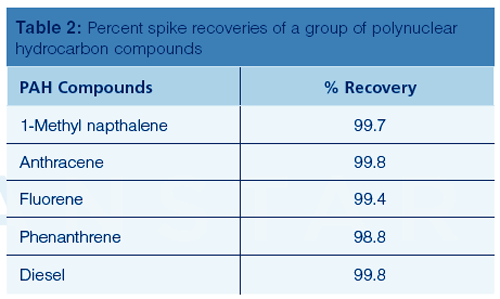
It should be noted that pyrene was also investigated at 325 °C, which resulted in a 90% recovery (a 99.3% recovery was achieved when the sample temperature was increased to 350 °C). However, since the goal of the study was to use a temperature of 325 °C for these experiments, pyrene was not investigated any further.
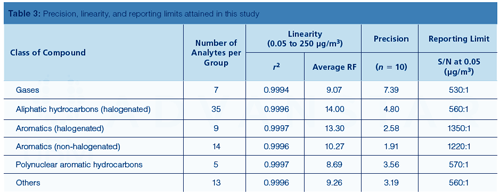
The data collected on target precision, linearity, reporting, and method detection limits are demonstrated in Table 3, which easily meets Method TO-17 performance criteria for the solid adsorbent sampling of ambient air (2). The reporting limits are calculated using a 1-L sample volume. The dynamic range achieved was at least four orders of magnitude across the target component list.
The results from the internal standard precision study are presented in Table 4.

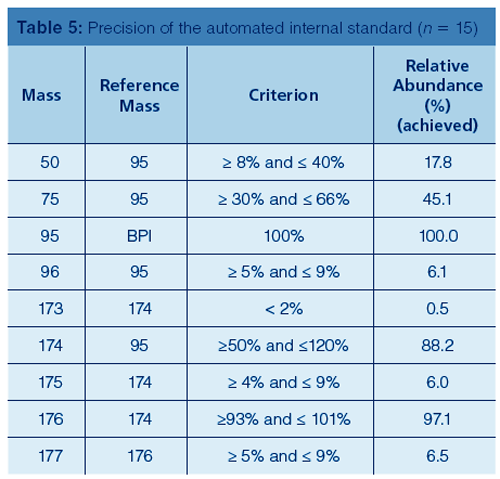
Table 5 displays the results for the BFB tune and the requirements set forth in EPA Method TO-17.
Discussion
Thermal desorption is a very cost effective and accurate technique for the sampling and analysis of air samples at very low detection capability. Thermal desorption has many environmental applications such as soil gas, studying healthy building syndrome, fenceline monitoring, and indoor–outdoor air analysis as well as addressing industrial hygiene concerns since 2009 (7). Sorbent tubes are small and light, making them easy to transport, thus reducing shipping costs compared to other techniques. In addition, the tubes are cleaned during the desorption process, rendering them available for immediate resampling, which can be verified with a short GC–MS analysis.
Water management is rigorous and automatic. Eliminating or reducing water entering the analytical system prevents the “quenching” of the response of target analytes, yielding accurate data and enhanced detection limits. Sample integrity is preserved using the following automated processes:
- A surrogate may be spiked onto the tube before sending the tube into the field for sampling.
- The sample tube and the concentrator trap is leak checked before desorption.
- An optional internal standard can be automatically spiked onto the tube.
- If needed, a tube impedance check can be performed on the tube to ensure packing is consistent.
- The sample may be recollected onto a new tube or the same tube if there is a need to reanalyze, or if the sample needs to be preserved for legal purposes.
Conclusion
In conclusion, the TO-17 method using the soil vapour intrusion sorbent tube designed for this investigation has demonstrated the capability of retaining the gaseous volatiles while extending the analyte range to phenanthrene. All US EPA method criteria were met and the management of moisture in the soil gas was adequately addressed. The achievable linear dynamic range of 0.05–250 µg/m3 was acceptable for the vast majority of soil samples encountered. If dilution is required, this can be accomplished by modifying the split ratios on the thermal desorber, or by recollection of the sample. The same calibration curve can be used and the dilution factor can be applied in the processing sequence. A reporting limit of 0.05 µg/m3 was achieved, which is below the required limit for regulatory agencies.
Acknowledgements
The authors would like to acknowledge the efforts of Luba Tsurikova and Patrick Novak of CARO Analytical Services, who were essential to the success of this project, together with the expertise of Roberta Provost of Pace Analytical Services, Jamie Brown of Supelco, and Ryan Sieber, Alan Gallaspy, Arlene Parces, and Martin Jacquez of PerkinElmer Inc.
References
- “How Much Air Do We Breathe?” (California Environmental Protection Agency, Air Resource Board, Research Note #94-11, 1994). Available at: http://www.arb.ca.gov/research/resnotes/notes/94-11.htm.
- “Guidance for Evaluating Soil Vapor Intrusion in the State of New York” (New York State Department of Health, Bureau of Environmental Exposure Investigation, Troy, New York, USA, 2006). Available at: https://www.health.ny.gov/environmental/investigations/soil_gas/svi_guidance/docs/svi_main.pdf.
- “Update on Contaminated Sites: Stage 6 Amendments to the Contaminated Sites Regulation” (British Columbia, Ministry of Environment. Victoria, BC, Canada, 2008). Available at: http://www.env.gov.bc.ca/epd/remediation/leg_regs/pdf/csr-stg-6-amend.pdf.
- Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air, “Method TO-15- The Determination of Volatile Organic Compounds (VOCs) in Air Collected In Specially-Prepared Canisters and Analyzed by Gas Chromatography/Mass Spectrometry (GC/MS)” (Office of Research and Development U.S. EPA, Cincinatti, Ohio, USA, 1999).
- Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air, “Method TO-17 Determination of Volatile Organic Compounds in Ambient Air Using Active Sampling onto Sorbent Tubes” (Center for Environmental Research Information, Office of Research and Development U.S. EPA, Cincinatti, Ohio, USA, 1999).
- R. Provost, L. Marotta, and R. Thomas, LCGC North Am.32(10), 810–818 (2014).
- L. Marotta, M. Snow, and S. Varisco, “Optimizing Analytical Parameters for Soil Vapor and Indoor Air Samples Using Automated Thermal Desorption/Gas Chromatography/Mass Spectrometry (ATD/GC/MS)” presented at Air and Waste Management Association (AWMA) Annual Symposium, Indianapolis, Indiana, 2009.
Lee Marotta is a GC and GC–MS Senior Application Scientist at PerkinElmer Inc., in Shelton, Connecticut, USA.
Stephen Varisco is Technical Manager of CARO Analytical Services in Richmond, British Columbia, Canada.
Miles Snow is a Principal Applications Scientist at PerkinElmer Inc.
Tom Kwoka is a Senior Product Specialist at PerkinElmer Inc.
Robert Thomas is the Principal Consultant at Scientific Solutions in Gaithersburg, Maryland, USA.

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.
Fundamentals of Benchtop GC–MS Data Analysis and Terminology
April 5th 2025In this installment, we will review the fundamental terminology and data analysis principles in benchtop GC–MS. We will compare the three modes of analysis—full scan, extracted ion chromatograms, and selected ion monitoring—and see how each is used for quantitative and quantitative analysis.
Quantifying Microplastics in Meconium Samples Using Pyrolysis–GC-MS
March 26th 2025Using pyrolysis-gas chromatography and mass spectrometry, scientists from Fudan University and the Putuo District Center for Disease Control and Prevention detected and quantified microplastics in newborn stool samples.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)