Evolving Interaction Polymer Chromatography
LCGC Europe spoke to André Striegel about the role and benefits of interaction polymer chromatography (IPC) for polymer analysis.
Q. Interaction polymer chromatography (IPC) is regarded as an “umbrella” term that covers a variety of separation techniques. Can you define IPC and describe what techniques this term includes?
A: The term “interaction polymer chromatography” or IPC encompasses gradient polymer elution chromatography (GPEC, in both traditional and interactive modes), temperature gradient interaction chromatography (TGIC), and barrier methods, such as liquid chromatography at limiting conditions of absorption, and desorption and SEC-gradient analysis.
Q. Is there a distinct retention mechanism for IPC techniques?
A: One generally distinguishing feature of almost all IPC methods is that the retention mechanism is predominantly governed by enthalpic interactions between the analyte and the column packing material, as mediated by the mobile phase and, in TGIC, also by temperature. This distinguishes IPC techniques from so‑called “size‑based” separations, such as size‑exclusion chromatography, hydrodynamic chromatography and flow field‑flow fractionation where retention is primarily a result of changes in the standard entropy of transfer between phases. An exception, which is still included under the IPC rubric, are liquid chromatography at the critical condition (LCCC) methods where, at least in theory, the entropic and enthalpic forces of transfer balance each other resulting in the standard Gibbs free energy of transfer between phases equalling zero.
Q. Are there specific types of applications that IPC techniques are used in, and what benefits do they offer?
A: IPC is primarily used to determine the chemical composition distribution of copolymers. The methods are also used to separate multi‑component mixtures according to the chemical composition of the samples’ constituents, to separate by tacticity, by end group distribution, and so on. Individually, they provide information not generally obtainable by size‑based techniques. More powerfully, they can combine with these latter techniques, in a two‑dimensional fashion, to provide, for example, the combined chemical composition and molar mass distributions (CCD×MMD) of a copolymer or blend in a single analysis.
Q. Can you describe an example that illustrates the benefits of IPC in practice?
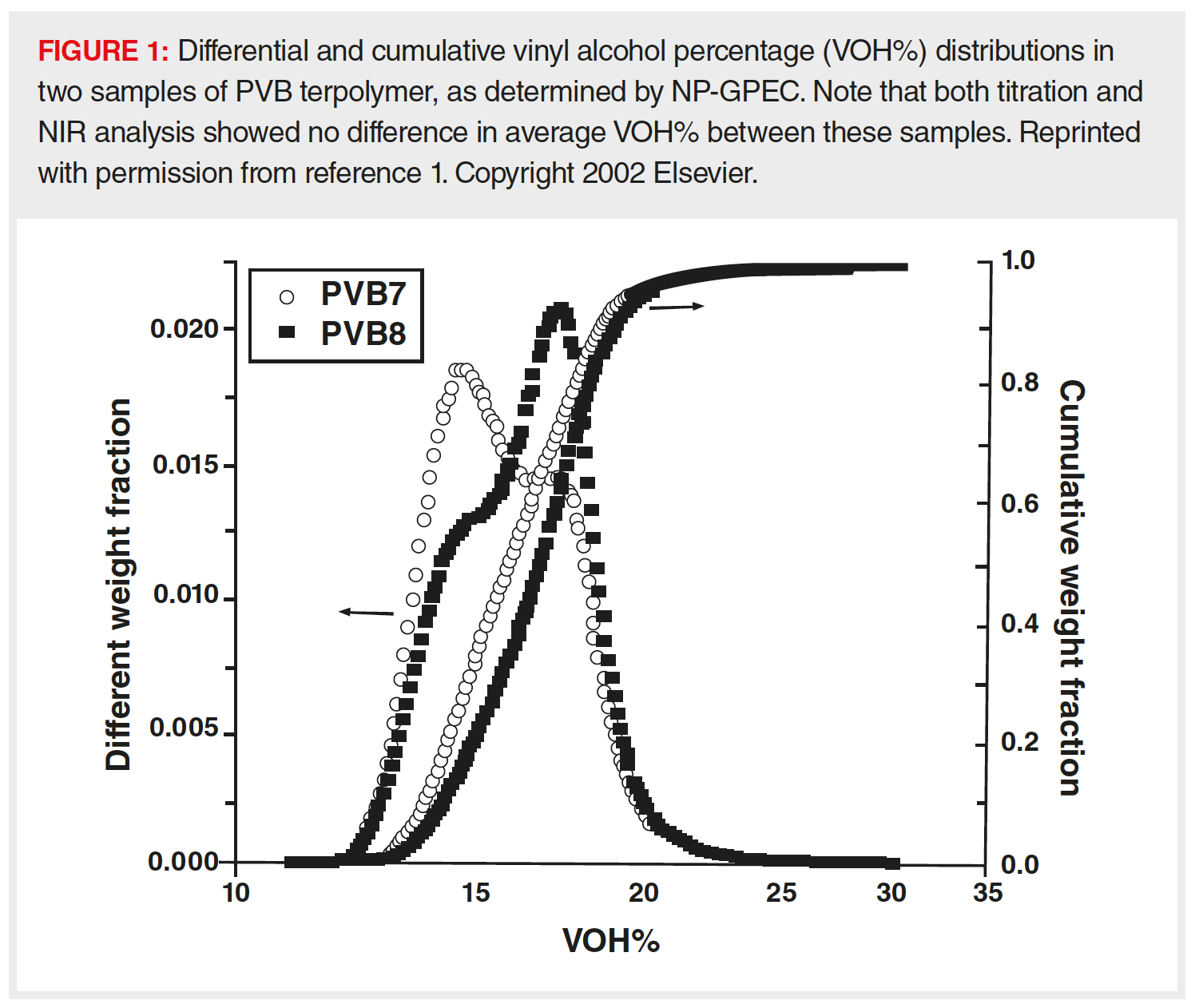
A: When I worked in the chemical industry, I developed an interactive GPEC method to determine the vinyl alcohol (VOH) distribution in poly(vinyl butyral) or PVB terpolymer (1). PVB is primarily used as the plastic interlayer in automotive and architectural safety glass because of its excellent penetration resistance and adhesion to glass, and the VOH content is a key parameter in controlling this adhesion; good adhesion prevents the glass on the inside of your windshield from flying into your face when a rock hits the windshield! There are existing titration, nuclear magnetic resonance- (NMR) spectroscopy, and near infra-red (NIR) spectroscopy methods to measure the VOH content, but all these only provide average values, whereas a chromatographic method, such as the normal-phase (NP) GPEC method developed, provides the entire VOH distribution and accompanying statistical averages and dispersity (1). For example, the two PVB samples in Figure 1 differed from each other with respect to their adhesive properties when, in theory, they were supposed to be identical in this regard. VOH content was the immediate suspect, but both titration and NIR analysis showed no quantitative difference in average VOH content. Employing NP-GPEC, the difference in performance behaviour of the samples could be immediately attributed to their different VOH distributions, shown in the overlays in Figure 1.
Q. IPC is more commonly used in research and specialized industrial laboratories. Why is this and do you think the techniques could be adopted more routinely? What are the main obstacles preventing this?
A: The biggest obstacle towards more widespread use of IPC methods is that little guidance has been provided in the literature with respect to method development for the various techniques. Most publications showcase what I refer to as “bespoke” methods, that is, they are applicable only to the analyte(s) studied in that particular paper. It is not clear how these particular bespoke methods were developed nor how they can be applied or adapted to the analysis of a different polymer. There is, however, ample potential for these techniques for the analysis of industrial polymers intended for both specialized and everyday applications because these are usually complex macromolecules or blends possessing distributions in a host of physicochemical properties.
Q. You recently published a paper on method development in IPC (2). What was the aim of this paper and what were the main conclusions?
A: My main aim was to address the shortcomings mentioned previously with respect to guidance in IPC method development. Essentially, I tried to provide a self‑contained guide to the fundamental chromatographic and polymer science principles that need to be considered when developing methods, as well as to provide guidance specific to each of the individual IPC methods mentioned above.
Q: Are there any specific differences that need to be taken into account when developing an IPC method compared to other approaches?
A: The biggest differences between IPC methods and size-based methods regard the use of mixed solvents in the former and, therefore, solvent gradients; the role of temperature and, in TGIC, temperature gradients the use of columns with a functionalized surface that interacts with the analytes. There is also the fact that one is often trying to separate a multi-component mixture containing many more components than are usually separated in SEC, HDC, or flow FFF. In the cases of solvent and temperature, one must understand (or, for temperature, continue to try to understand because systematic studies of the effect of temperature on IPC separations other than TGIC are almost non-existent) how these mediate interactions between analytes and stationary phase chemistry to achieve preferential retention. Column functionality, gradient design, and multi-component separations all involve a knowledge of fundamental separation science greater than is usually needed when designing a size-based separation.
Q. How do you see IPC evolving?
A: I would very much like to see these methods in the hands of a more general, yet simultaneously more applied, audience, such as industrial non-specialists. As method development thrives, so will the information obtained and benefits derived there from, in terms of superior products and more versatile applications. To do this, non-specialists need to know where to start with respect to method development. Another thing that is needed is columns specific to IPC. Currently, except for when size‑exclusion chromatography columns can be repurposed for IPC analysis, IPC employs columns designed for small‑molecule separations. There is great product variability amongst manufacturers of what should be the same columns; for example, the performance of diol- or cyano columns from different manufacturers can vary greatly when the columns are used in interactive GPEC or other IPC analyses. Other issues such as surface inhomogeneities in “small‑molecule” columns can compromise macromolecular analyte recovery and lead to biased results regarding molar mass, chemical composition, and so on.
References
- A.M. Striegel, J. Chromatogr. A. 971(1–2), 151–158 (2002).
- A.M. Striegel, TrAC 130, 115990 (2020).
Further Reading
- W. Radke, J. Chromatogr. A., 1335, 62–79 (2014).
- Y. Brun and C.J. Rasmussen, in: Liquid Chromatography: Fundamentals and Instrumentation, S. Fanali, P.R. Haddad, C.F. Poole, and M.-L. Riekkola, Eds. (Elsevier, Amsterdam, 2nd ed., 2018) pp. 275–318.
- W. Staal, Ph.D. Dissertation, “Gradient Polymer Elution Chromatography”, Eindhoven University of Technology, Netherlands (1996).

André Striegel is a scientific advisor in the Chemical Sciences Division of the Material Measurement Laboratory at the National Institute of Standards & Technology (NIST). He received his Ph.D. in analytical chemistry in 1996 and his B.S. in chemistry in 1991, both from the University of New Orleans (USA). From 1996 to 1998 he performed postdoctoral research for the U.S. Department of Agriculture, at the National Center for Agricultural Utilization Research. For the next six years he worked for Solutia (now Eastman Chemical), achieving the rank of Research Specialist. From 2004 to 2011 he was assistant professor of both analytical and materials chemistry in the Department of Chemistry and Biochemistry at Florida State University (FSU, USA). In September 2011 he joined NIST. His research interests are in the area of polymer characterization, in particular applying separation science to determining structure‑property relations of complex macromolecules, and in the fundamental aspects of separation and detection methods.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Quantifying Terpenes in Hydrodistilled Cannabis sativa Essential Oil with GC-MS
April 21st 2025A recent study conducted at the University of Georgia, (Athens, Georgia) presented a validated method for quantifying 18 terpenes in Cannabis sativa essential oil, extracted via hydrodistillation. The method, utilizing gas chromatography–mass spectrometry (GC–MS) with selected ion monitoring (SIM), includes using internal standards (n-tridecane and octadecane) for accurate analysis, with key validation parameters—such as specificity, accuracy, precision, and detection limits—thoroughly assessed. LCGC International spoke to Noelle Joy of the University of Georgia, corresponding author of this paper discussing the method, about its creation and benefits it offers the analytical community.









