Equine Drug Testing with LC–MS/MS
Doping and cheating the competition may be as old as competition itself. Doping horses for chariot racing was reported during the Roman Empire and doping is now commonly reported in the press. Advanced technologies such as ultrahigh-pressure liquid chromatography (UHPLC) analysis coupled to ultrafast tandem mass spectrometry (MS/MS) detection provide excellent sensitivity for the analysis of horse doping agents.
Photo Credit: Colormos/Getty Images

Gesa Johanna Schad, Shimadzu Europa, Duisburg, Germany.
Doping and cheating the competition may be as old as competition itself. Doping horses for chariot racing was reported during the Roman Empire and doping is now commonly reported in the press. Advanced technologies such as ultrahigh-pressure liquid chromatography (UHPLC) analysis coupled to ultrafast tandem mass spectrometry (MS/MS) detection provide excellent sensitivity for the analysis of horse doping agents.
Faster, higher, further - doping has been present in competitive sports events since the Olympic Games, held in Ancient Greece. But back then, it was not possible to detect or prove the abuse of performance enhancing substances. The first public case of illegal doping was reported in 1812 - and it was only discovered because the culprit was caught in the act (1).
Doping involves the use of banned performance-enhancing drugs or the use of banned methods to improve performance. However, doping does not always refer to drug abuse to improve an athletic effort. In horse racing, for example, negative doping is also an issue, where the animal is treated to ensure poor performance.
The motto “If it can’t be detected, it’s allowed” predominated in the past. Nowadays, improved detection methods using the latest analytical techniques enable determination of even trace amounts of doping agents in blood and urine. The instrumentation available in testing laboratories is therefore crucial for quality of data obtained from drug tests in different matrices. This article presents the advantages of ultrahigh-pressure liquid chromatography (UHPLC) analysis coupled to ultrafast tandem mass spectrometry (MS/MS) detection for excellent sensitivity in the analysis of horse doping agents. In the past older analytical techniques were used, with qualitative analysis with colour indicators, etc. They were the only methods available then and were less specific and less sensitive. LC–MS/MS equipment is becoming more affordable and is therefore more commonly used today.
Experimental
After sample pretreatment, urine samples from a horse doping laboratory were tested for various steroid hormones in the form of free steroids or steroid esters. Small molecules such as banned neuroleptics, benzodiazepines, and opioids were also investigated. The samples were analyzed using a triple quadrupole mass spectrometer coupled to a UHPLC system operating in scheduled multiple reaction monitoring mode (MRM) mode with fast polarity switching (5 msec) for the detection of positively and negatively charged ions in one run. To corroborate the data quality of the ultrafast scheduled MRM analysis, two different screening methods containing MRM transitions for either 13 components (resulting in 26 MRMs) or 127 components (resulting in 254 MRMs) were compared. The repeatability of MRM-only experiments compared to MRM experiments including a synchronized survey scan (data dependent product ion scan) was also investigated.
Analytical Conditions: Liquid chromatography: Nexera X2 UHPLC system (Shimadzu) consisting of two LC-30AD solvent pumps, SIL-30AC autosampler and CTOâ30A column oven; analytical column: 2.1 mm × 100 m, 1.7-μm Acquity C18 (Waters); mobile phase A: water + 0.1% formic acid; mobile phase B: acetonitrile + 0.1% formic acid; flow rate: 0.35 mL/min; gradient conditions: 0.00 min 5 %B; 1.50 min 5 %B; 2.30 min 28 %B; 6.50 min 36 %B; 7.00 min 100 %B; column temperature: 25 °C; injection volume: 10 μL
Mass Spectrometry Detection: LCMSâ8050 triple quadrupole mass spectrometer (Shimadzu); ionization: HESI (positive/negative); nebulizing gas flow: 3.00 L/min (N2); drying gas flow: 5.00 L/min (N2); heating gas flow: 15.0 L/min (air); DL temperature: 250 °C; block temperature: 450 °C; interface temperature: 300 °C
Results
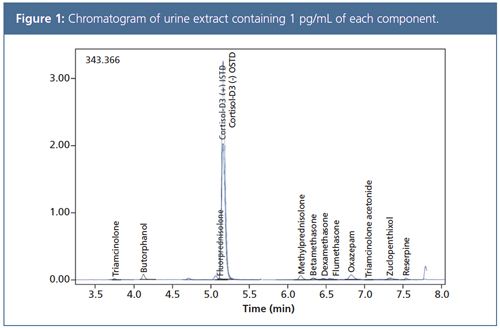
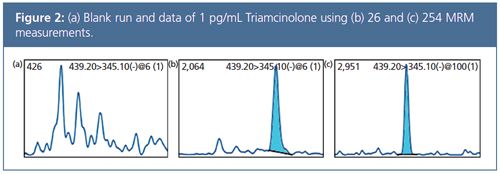
Data Comparison: The chromatogram shown in Figure 1 was obtained from a urine extract containing 1 pg/mL of 13 different components. Comparison of data quality measured with 13 events (26 MRM) and 127 events (254 MRM), using the compound Triamcinolone as an example, showed that high speed analysis with 1 ms dwell time and ultra-fast polarity switching resulted in no loss of sensitivity (Figure 2).
In the next experiment, repeatability of the assay was tested. A standard solution equivalent to an extracted sample at 2 pg/mL was injected six times. An acquisition method using MRM mode only was compared with an acquisition mode combining MRM mode with a synchronized survey product ion scan at 30,000 Da/s. The synchronized survey scan is not just limited to product ions, it is also possible to define specific masses in the method that will trigger a scan.
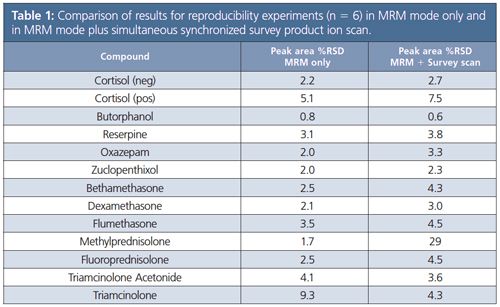
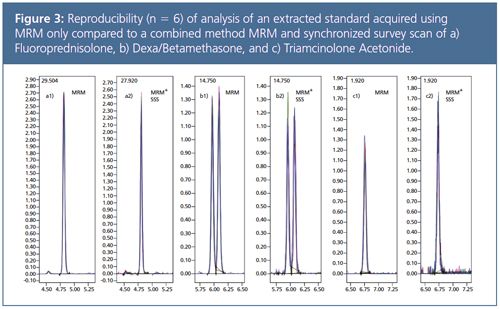
Table 1 shows a comparison of results for reproducibility experiments (n = 6) in MRM mode only and then analyzed again in MRM mode with a dwell time of 5 msec for all components plus simultaneous synchronized survey product ion scan (scanning speed 30,000 amu/sec). Concentration of the injected standard is equivalent to an extracted sample with a concentration of 2 pg/mL. This comparison confirmed that simultaneous measurement of quantitative (MRM) and qualitative (synchronized survey scan) data is possible without significant loss in sensitivity or reproducibility (see Figure 3).
Conclusion
A robust, selective and sensitive analytical method for the simultaneous determination of various illegal performance enhancing drug substances was developed. Independently of the number of MRMs or synchronized survey scans performed simultaneously, LC–MS/MS offered good sensitivity with high data quality in scheduled MRM mode with fast polarity switching for the detection of positively and negatively charged analytes in one run. MRM offers more specific information on a sample compound for more precise sample identification. It can detect and help to identify unknowns.
Regular doping controls using modern highâend analytical instruments make life difficult for doping offenders in equestrian sports. Statements such as “If it can’t be detected, it’s allowed” are outworn and outpaced by the capabilities of new analytical techniques.
References
- Liam O’Brien, What’s the SP?: Betting on Racing: An A-Z (eBook, 2014).
Gesa Johanna Schad graduated with a Diploma in chemical engineering from the Technical University NTA in Isny, Germany, in 2004 and as a Master of Science in pharmaceutical analysis from the University of Strathclyde in Glasgow, UK, in 2005. She worked until 2006 as a consultant in HPLC method development and validation in an analytical laboratory of the FAO/IAEA in Vienna, Austria. She gained her doctorate for research in pharmaceutical sciences at the University of Strathclyde in 2010 and was employed as a HPLC specialist in the R&D department at Hichrom Ltd. in Reading, UK from 2009. Since 2013, she has worked as a HPLC product specialist and since 2015 as HPLC Product Manager in the analytical business unit of Shimadzu Europa in Duisburg, Germany.

Distinguishing Alcohol- from Non-Alcohol-Associated Liver Cirrhosis with LC-MS
May 7th 2025A pilot study investigating whether nicotinamide adenine dinucleotide kinase (NADK) expression is selectively diminished in alcohol-associated liver cirrhosis (AC), as well as evaluating its potential as a biomarker for this condition, measured AC and non-AC (NAC). Nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide phosphate (NADP+) levels in human liver samples were measured using liquid chromatography-mass spectrometry (LC-MS).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








