Ensuring Protein Reagent Quality by SEC-MALS
Quality and consistency in reagents is critical to successful drug discovery and development. When targeting a particular protein of interest, in vitro experiments should be performed with proteins of biological properties similar to those for in vivo tests. It is important that molecularity, purity, shape, and degree of heterogeneity remain the same when any alterations are made to the model protein or the formulation buffer. Multi-angle light scattering (MALS) combined with size-exclusion chromatography (SEC-MALS) is a very useful technique to monitor the solution properties of the protein as changes to reagents are made.
Wyatt Technology
Quality and consistency in reagents is critical to successful drug discovery and development. When targeting a particular protein of interest, in vitro experiments should be performed with proteins of biological properties similar to those for in vivo tests. It is important that molecularity, purity, shape, and degree of heterogeneity remain the same when any alterations are made to the model protein or the formulation buffer. Multi-angle light scattering (MALS) combined with size-exclusion chromatography (SEC-MALS) is a very useful technique to monitor the solution properties of the protein as changes to reagents are made.
As an example, crystallization studies typically have a higher rate of success when the proteins involved are simplified, for example, truncated or expressed in bacteria to minimize post-translational modifications. However, these forms may not be amenable to traditional characterization such as plate-based assays and certain biophysical techniques, where native protein is preferred. Affinity tags or changes to buffer excipients may also be required. All of these differences increase the likelihood of altering solution properties.
The example in this note shows how light scattering data obtained with a DAWN® MALS detector and analyzed by ASTRA® software (both from Wyatt Technology, Santa Barbara, California, USA) were used to elucidate the solution properties of protein expressed from two different constructs. Construct A is the shorter of the two, designed for crystallization efforts.
Figure 1: Two protein constructs: A (45 kDa) and B (46 kDa). SDS-PAGE shows similar purity.
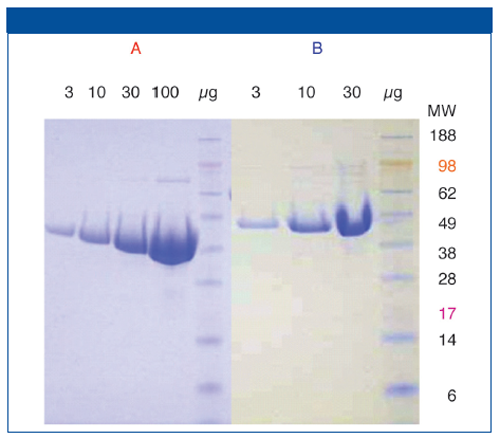
Presented with data from Figure 1, combined with knowledge that the enzyme showed activity and was the predominant species in mass spectrometry, one might not question the suitability of this reagent. SEC-MALS data (Figure 2), however, show that this enzyme is in fact quite heterogeneous.
Figure 2: Standard chromatographic trace (UV 208nM) with MALS data (linear traces within the chromatograms represent the mass distribution across the peak). The enzyme from construct B is much more homogeneous, and is in its biologically active form of a dimer (89 KDa).

Construct B addressed this; it is the full-length enzyme. MALS data show that the protein is in its biologically active form (dimer)and is highly homogeneous. This information gave the project team confidence to move forward with this construct for crystallography, NMR, and high-throughput screening (HTS) efforts.
This note graciously submitted by Mark Tardie and George Karam; Pfizer Central Research, Groton, Connecticut, USA.

Wyatt Technology
6330 Hollister Avenue, Santa Barbara, California 93117, USA
Tel. +1 (805) 681 9009
Website: www.wyatt.com • E-mail: info@wyatt.com
A Matrix-Matched Semiquantification Method for PFAS in AFFF-Contaminated Soil
Published: April 14th 2025 | Updated: April 14th 2025Catharina Capitain and Melanie Schüßler from the Faculty of Geosciences at the University of Tübingen, Tübingen, Germany describe a novel approach using matrix-matched semiquantification to investigate per- and polyfluoroalkyl substances (PFAS) in contaminated soil.
Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.











