DAR Analysis of Antibody–Drug Conjugates
In this application note, an antibody–drug conjugate (ADC) was analyzed using a TSKgel® Butyl-NPR column, the least hydrophobic of the TSKgel HIC columns. Both unconjugated and drug conjugated Trastuzumab samples were successfully separated with baseline resolution. The baseline resolution enabled an easy integration and quantification of different drug pay loads in ADC characterization.
Tosoh Bioscience GmbH
In this application note, an antibody–drug conjugate (ADC) was analyzed using a TSKgel® Butyl-NPR column, the least hydrophobic of the TSKgel HIC columns. Both unconjugated and drug conjugated Trastuzumab samples were successfully separated with baseline resolution. The baseline resolution enabled an easy integration and quantification of different drug pay loads in ADC characterization.
In antibody–drug conjugates (ADCs) a cytotoxic drug is chemically bonded to an antibody (IgG). Because antibodies have numerous binding sites for a low-molecular-weight drug, heterogeneity arises with respect to the number of bonded drug molecules and the binding sites. Since low molecular drugs are more hydrophobic than monoclonal antibodies, differences in hydrophobicity arise when the numbers of bonded drug molecules differ. This property can be utilized to determine the drug-to-antibody ratio (DAR) by hydrophobic interaction chromatography (HIC).
Material and Methods
Column: TSKgel Butyl-NPR, 4.6 mm × 10 cm L
Mobile phases: A) 25 mmol/L phosphate buffer (pH 7.0) including 1.5 mol/L ammonium sulphate
B) 25 mmol/L phosphate bufffer (pH 7.0)/2-propanol = 8/2
Gradient: 0100% B (20 min)
Flow rate: 0.5 mL/min
Detection: UV @ 280 nm
Injection volume: 10 μL
Sample: Trastuzumab (0.24 mg/mL), ADC (Trastuzumab-vcMMAE, 2.2 mg/mL)
Results
An ADC (Trastuzumab-vcMMAE) in which an antineoplastic drug (monomethyl auristatin E, MMAE) is bonded via a linker to Trastuzumab was analyzed by hydrophobic interaction chromatography using a TSKgel Butyl-NPR column. By optimizing the organic solvent concentration in solvent B, peaks exhibiting different DARs (DAR = 0 to 8) could be separated well. To confirm the DAR, each peak was fractionated and attributed by liquid chromatography tandem mass spectrometry (LC–MS/MS) (data not shown).
Figure 1: HIC analysis of unconjugated Trastuzumab and drug conjugated Trastuzumab.
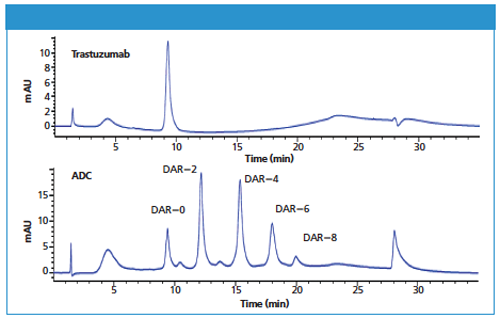
Both samples, unconjugated monoclonal antibody (Trastuzumab) and drug conjugated Trastuzumab (Trastuzumab-vcMMAE), were analyzed with a 4.6 mm × 10 cm, 2.5-μm TSKgel Butyl-NPR column. The unconjugated Trastuzumab was eluted as a major single peak (Figure 1, upper panel). The chromatogram of the drug conjugated Trastuzumab exhibited well resolved peaks with different retention times than that of the unconjugated antibody (Figure 1, lower panel). These well resolved peaks represent different drug-to-antibody ratios ranging from 0 to 8. The lower drug-loaded, less hydrophobic peaks elute first and the higher drug-loaded peaks elute later.
Conclusion
TSKgel Butyl-NPR, the least hydrophobic of the TSKgel HIC columns, is the best choice for efficient separation of antibody–drug conjugates. The 2.5-µm non-porous particles can be used with both conventional high pressure liquid chromatography (HPLC) and ultrahigh-pressure LC (UHPLC) systems.

Tosoh Bioscience GmbH
Im Leuschnerpark 4 64347 Griesheim, Darmstadt, Germany
Tel: +49 6155 7043700 Fax: +49 6155 8357900
E-mail: info.tbg@tosoh.com
Website: www.tosohbioscience.de
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).











