Effects of Sample-Dissolving Solvents and Sample Ionic Strength in Routine CE Operation
LCGC North America
The ionic strength of a sample and the solvent used to dissolve it play a pivotal role in routine capillary electrophoresis (CE).
What impact can choice of solvent have on the separations obtained in CE?
Sample diluent ionic strength, pH, organic solvent composition, and viscosity can all have a significant impact on the performance of CE separation. It is, therefore, essential that the choice of solvent is optimized during method development. The optimized diluent should be fully specified in the method and used consistently in method validation and all subsequent applications of the method.
What is the Effect of Sample Diluent Ionic Strength?
In CE the typical length of an injection inside the capillary is a few mm. This is a significant length given that the total capillary length may be 30 cm and the detection window may be 0.1 mm. The peak width is directly related to the injection zone length. Ideally the injection zone length would be very small but this would result in sensitivity issues.
There are many different in-capillary concentration approaches in CE that can be used to improve method sensitivity and separation efficiency by reducing peak width after injection. These have been summarized and interested readers should read these more in-depth publications (1,2).
The most common approach to reducing the zone length of the sample injection is by using a process termed "stacking." Stacking reduces the width of the sample zone at the start of the separation when the voltage is initially applied and results in an improved sensitivity (because the sample becomes more concentrated inside the capillary) and increased peak efficiency.
"Failure to use the exact sample diluent can lead to irreproducible separations and/or poor quantitative performance."
Stacking occurs when the sample is dissolved in a lower ionic strength solution than the separation electrolyte. Under these circumstances the field strength is higher in the sample zone than in the rest of the capillary, which is filled with electrolyte (Figure 1). The sample ions move forward rapidly in the sample zone until they encounter the electrolyte boundary where they experience a lower applied field and their migration rate slows down. In this way the sample zone is focused/condensed/concentrated and stacking can lead to as much as a 10-fold reduction in the starting peak width. This process is optimized if the sample is dissolved in a 1:10 dilution of the run electrolyte.
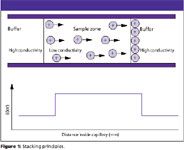
Figure 1
Figure 2 illustrates the stacking principle in action. Figure 2(a) is the separation achieved for a 0.5 mg/mL solution of a drug dissolved in 50 mM phosphate buffer. Separation was performed using the same 50 mM phosphate buffer. The peaks in the separation are relatively wide, which gives poor sensitivity and limited resolution. Figure 2(b) shows the stacking effect — in this instance a 0.5 mg/mL solution of the same drug batch was dissolved in a 5 mM phosphate buffer and separated with a 50 mM buffer.

Figure 2
The sample is concentrated within the capillary to give both smaller peak widths and higher sensitivity. This concentration results in better sensitivity as a result of taller peaks and better resolution because peaks are more efficient/thinner.
Stacking can also be deliberately performed if the solute is zwitterionic (that is, it has a positive charge at low pH and a negative one at high pH) (1). For example, samples can be deliberately prepared in a high pH diluent and separated using a low pH buffer. In this instance the samples would be negatively charged and would move to the back of the sample zone when the voltage is initially applied. When the sample ions reach the back of the zone they protonate and move forward and the sample zone length is greatly reduced.
What Should I Do if my Samples Have a High Ionic Strength?
If the sample diluent has a higher ionic strength than the running buffer then destacking or anti-stacking occurs and separation performance is heavily reduced. The presence of high ionic strength can lead to heavily distorted peak shapes and loss of separation efficiency (3). This situation may occur, for example, when samples that are saline isotonic are analyzed (that is, 0.9 M NaCl).
Increasing the ionic strength of the buffer reduces the destacking effects but generates high operating currents. The use of a combination of short-end injections and high buffer concentration has been shown to be successful for high ionic strength samples (3). Another approach is to add zwitterionic buffer ions to the buffer to increase ionic strength. Zwitterionic additives increase the ionic strength of the buffer but do not increase the operating currents (4).
Addition of organic solvents to the sample solution can be used to suppress ionic strength and reduce/eliminate destacking effects. Acetonitrile is a particularly useful solvent to use because this suppresses ionic strength but also has a lower viscosity than water (5). Acetonitrile addition, therefore, achieves a stacking effect because ions move faster in a less viscous solution. Yet another approach is to stack cations in high ionic strength solutions by immediate injection of an acid after injection of the sample solution before application of the separation voltage (6).
Sample preconcentration using optimized sample diluents has been heavily studied in micellar electrokinetic chromatograpy (MEKC) (7). MEKC uses charged micelles to achieve chromatographic separations of solutes. "Sweeping" approaches have been described where sample concentrations can be increased in-capillary by the order of 1–1000-fold. Long injection times are performed and the charged micelles move (sweep) through the sample zone collecting the sample molecules into a tight concentrated portion at the back of the sample zone.
Can I Dissolve my Sample in Pure Water or 100% Solvent?
Organic solvents are often used for the analysis of insoluble solutes or to solubilize samples with a complex insoluble matrix such as a cream. However, they can have a dramatic effect on separation. If the sample is dissolved in pure water (or solvent) then the injection zone has a high resistance and prevents the free passage of current.
This will lead to an irreproducible current trace and variability in migration times. If a large injection volume/time is used with a sample dissolved in pure solvents then the current is unable to pass along the capillary and the CE equipment senses a fault and turns itself off. If pure solvent or water is used then minimal injections should be performed.
It should also be noted that if mixtures of solvent and water or buffer are used as diluent then air bubbles can form when the voltage is applied (8). Heat is generated in the sample zone, which causes out-gassing in the samples. These air bubbles will prevent a full electric circuit and the equipment will shut down during the initial voltage application phase after injection. This likelihood of shutdown is reduced with shorter injection times and high ratios of water:solvent (for example, 50:50 v/v) (8). Samples can also be sonicated/filtered to remove dissolved gases to reduce this occurrence.
Microemulsion CE is an expanding CE technique, which uses microemulsions as the separation media (9). Solvents severely disrupt separations because they destroy the microemulsion droplets — it is, therefore, common to use microemulsion to dissolve the samples. For example, 100% microemulsion was used as the dissolving solvent in validation work on a method for determining atropine impurities (10).
Micelles are also destroyed by the presence of excessive organic solvent. Disastrous effects on the separation can occur if the organic solvent content is altered in MEKC methods. The effect of methanol addition on MEKC methods is shown in Figure 3(a)–(c). Figure 3(a) shows the acceptable separation of a test mix prepared in water using a MEKC method with a buffer containing 75 mM SDS to form the micelles (11). Separation of an identical sample solution except containing 30% methanol produces a very poor electropherogram [Figure 3(b)]. Using a much greater concentration of sodium dodecyl sulphate (SDS) (100 mM) generates more micelles and allows the more acceptable use of 30% methanol in the sample [Figure 3(c)]. Therefore, the sample diluent should not be varied in MEKC methods and should be identical for both standards and samples.

Figure 3
Organic solvents are generally volatile and will evaporate. CE sample vials are often not completely sealed or the seal is punctured, which allows volatile solvents to escape. As solvent evaporates the sample will become more concentrated and inaccurate assay values will occur. In addition if several standards are analyzed during an extended sequence then response factors will drift upward. Table I shows the evaporation rates encountered in routine CE operations (8). Internal standards (ISs) solve these issues as they become more concentrated in the samples at the same rate as the samples or standards.
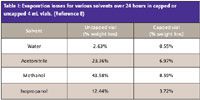
Table I: Evaporation losses for various solvents over 24 hours in capped or uncapped 4 mL vials.
Selection of sample diluent are especially important in CE–MS where ionization, solvent volatility, and sample solubility are affected by solvent (13).
Alternatives to organic solvents include cyclodextrins or urea dissolved in the water (or dilute buffer) because these additives solubilize water-insoluble compounds. These additives are not volatile so do not suffer evaporation problems. However, there are additional issues such as additive purity, equipment cleanliness problems, and cost to consider regarding their use.
Water-insoluble compounds that are ionizable may also be readily dissolved in pH-adjusted water (for example, water adjusted to pH 2 with concentrated phosphoric acid). The stability of the sample solution should be carefully assessed using this approach.
Does Sample Solvent Viscosity Have an Effect?
Viscosity has a profound effect on the amount loaded during pressure injection. There is a viscosity factor in the loading equation:

It is essential to match the viscosity of the sample and standards, otherwise differential loading amounts and incorrect quantitative results will occur. For example if the viscosity of samples solutions is higher than that of the standards then low assay results will occur. An increased sample solution viscosity can occur because of the nature of the sample under test, for example, tablets often contain cellulose which thickens solutions when dissolved.
Figure 4 shows the quantitative effect of varying sample solution viscosity (8). Different amounts of sucrose were weighed into aliquots of the same sample solution. The sucrose increased the viscosity leading to decreased amounts of sample introduced into the capillary with correspondingly lower peak areas (Figure 4). For example, the addition of 500 mM of sucrose reduced the peak area to a third of the original amount, which would generate to a 33% recovery result if assessed during method validation.

Figure 4
Fortunately, the sample also contained an IS. Peak area ratios were, therefore, unaffected as the peak areas of both the internal standard and sucrose decreased to the same extent. Therefore, as previously described (12), ISs should be used whenever possible in CE to minimize sample effects and improve quantitative analysis performance.
Another approach to overcome viscosity issues can be the addition to directly mimic the sample. For example, in the determination of alginate in pharmaceutical preparations the authors added placebo tablets into standard solutions to ensure that the viscosity of the standard solutions exactly matched the samples — recoveries of 99.1% were obtained using this approach (14).
How Should I Approach Method Development?
If possible request any details of sample stability, pKa and solubility in different solvents and pH ranges. Information on solvents used in HPLC can also be useful.
If possible initially assess the use of a 1:10 dilution of the running buffer as the optimum choice — this maximizes stacking effects but still maintains stable current traces and avoids the possibility of injection/equipment failures.
A quick test of solvent applicability is to prepare samples in various solvents. A few drops of each sample solution can be added to aliquots of buffer. If the buffer goes cloudy when the drops are added then the sample has precipitated in the buffer and this solvent is probably a poor choice.
"Always identically match the viscosities of standard and sample solutions or use an internal standard."
Always work on real samples. It may be possible to develop a separation using standards dissolved in a particular solvent. However, there may be extraction difficulties with real samples, for example, tablets, which require the use of a different solvent and this new solvent often disrupts the separation previously achieved.
What Do I Need to Cover During Method Validation?
This largely depends on the final optimized diluent that is included in the method. For example, if the final method states 5 mM phosphate pH 2.3 as the diluent — it may be appropriate to assess diluent in the range 4–6 mM and pH 2.1–2.5 to gain confidence in performance and allow some flexibility during sample diluent preparation. System suitability samples should be run prepared in diluents covering the ranges being assessed. Once the limits have been scientifically proven to be acceptable then they can be included in the method, for example, to prepare samples in 4–6 mM phosphate at pH 2.1–2.5.
Robustness experiments should be conducted using appropriate samples and/or standards to test the impact of diluent composition variations and to determine operating limits.
Attention to sample stability in the optimized dissolving solvent would need to be tested to experimentally determine a shelf-life for samples.
Kevin Altria is the editor of CE Currents for LCGC Europe and is senior principal scientist at GlaxoSmithKline, Harlow, Essex, UK and is a member of the Editorial Advisory Board of LCGC Europe, Advanstar House, Park West, Sealand Road, Chester, UK, CH1 4RN, UK.
References
(1) Z.K. Shihabi, J. Chromatogr. A, 902(1), 107–117 (2000).
(2) M.C. Breadmore, Electrophoresis, 28(1–2), 254–281 (2007).
(3) K.D. Altria, B. Clark and T. Kelly, Chromatographia, 43(3–4), 153–158 (1996).
(4) E. Stellwagen, J.D. Prantner and N.C. Stellwagen, Analytical Biochemistry, 373(2), 407–409 (2008).
(5) M.A. Friedberg, M. Hinsdale and Z.K. Shibabi, J Chromatogr. A, 781(1–2), 35–42 (1997).
(6) D.J. Weiss, K. Saunders and C.E. Lunte, Electrophoresis, 22(1), 59–65 (2001).
(7) S.L. Simpson, J.P. Quirino and S. Terabe, J. Chromatogr. A, (2008) in press.
(8) K.D. Altria and F. Campi , LCGC Eur., 13(1), 16–23 (1999).
(9) P.E. Mahuzier et al., LCGC Eur., 16(1), 22–29 (2003).
(10) Y. Bitar and U. Holzgrabe, J. Pharm.Biomed. Analysis, 44(3), 623–633 (2007).
(11) K.D. Altria, B. Clark and M.A. Kelly, J. High Resol. Chrom., 22, 55–58 (1999).
(12) K.D. Altria, LCGC Eur., 15(9), 588–594 (2002).
(13) A.M. van Wijk, et al., J.Chromatogr. A, 1159(1–2), 175–184 (2007).
(14) N. Oztekin, S. Baskan and F.B. Erim, J. Chromatogr. B, 850, 488–492 (2007).

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.






