Effects of Buffer Capacity in LC, Part II: Visualization of pH Changes Inside the Column
LCGC North America
In situ measurements of the mobile-phase pH before and after the column help to rationalize the effects of mismatch in pH and concentration between the mobile phase and sample buffer mismatch in reversed-phase LC separations.
If I inject a sample buffered at a pH different from that of the mobile phase, how quickly is the sample buffer neutralized inside the column?
In last month’s installment of “LC Troubleshooting,” we kicked off a series of articles on the topic of buffer capacity and LC mobile phases. A fair amount has been written on this topic in LCGC over the years (1–4), but over time we continue to learn more about the details of what goes on inside LC columns, and thus it is good to periodically refresh our view of how buffers affect our separations.
Last month (5), we demonstrated how the choice of mobile-phase buffer concentration and sample injection volume can have a dramatic impact on the quality of the resulting separation in cases where the analytes of interest are ionogenic and the mobile phase and sample pH values fall on two sides of a pKa of an analyte. For the purpose of demonstration, we used the analyte benzoic acid with the non-ionogenic analyte benzyl alcohol serving as a control molecule. This month, we take this line of thinking one step further, and use in situ measurements of the mobile- phase pH before and after the column to help rationalize the effects of the mobile phase-sample buffer mismatch on the separations that we observe.
Background: In situ Determination of pH in LC Systems
A few years ago, we developed an approach to determine the pH of flowing mobile phase at the column inlet and outlet as a means of better understanding how pH changes inside of LC columns during separations (6). Our approach relies on the response of colorimetric indicator dyes to changes in pH. There are other approaches available to measure the pH of flowing mobile phase in an LC system, such as ion selective electrodes based on glass membranes built into a flow-through cell. We developed the colorimetric approach mainly because it has two features that differentiate it from electrode-based approaches: 1) the colorimetric approach can be used at high pressures; and 2) it enables detection of very fast changes in pH (on the scale of at least 10 ms). An illustration of the instrument setup for the colorimetric approach is shown in Figure 1. Panel a shows the configuration of the system used both for calibration and for in situ determination of mobile-phase pH at the column inlet. The central idea is that a cocktail of pH indicator dyes (commercially available in liquid form as “universal pH indicator”) is added to the mobile phase (1 part indicator, 10 parts mobile phase) immediately prior to a diode-array UV detector that enables rapid measurement of the absorbance spectrum of the dye mixture over time. We first calibrate the system by delivering known ratios of two buffers (in the case of this work, pH 2.5 and 7.5) for which the expected pH can be calculated, and measuring the UV-visible absorbance spectrum at each of these pH levels. Absorbance values from three wavelengths (red, green, and blue light) are then used to calculate a single “hue” value that can be related to pH. This hue value provides a numerical position around the color wheel, as shown in Figure 1. This process produces a kind of calibration function. We start with known pH levels, and calculate the hue. Then, in an experiment where we want to know an unknown pH level, we measure the hue and infer the pH from the calibration function. Readers interested in the details of these steps are referred to our prior paper describing the approach (6).
Figure 1: Instrument setup for determining mobile phase pH in situ. (a) configuration for determining pH at the column inlet; (b) configuration for pH determination at the column outlet.
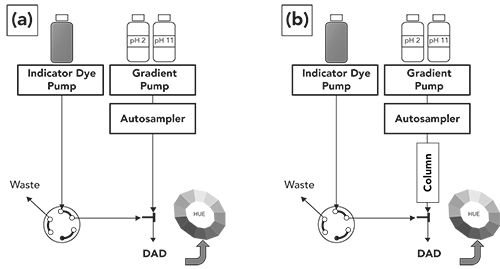
As indicated above, the configuration shown in Figure 1a enables calibration of the system, and determination of mobile-phase pH at what is effectively the column inlet (for instance, after the sample injector, but before the detector). Note that in this case we don’t actually place the column in the system; doing so would result in the indicator dyes flowing through the column and creating an entire set of challenges that is not necessary here. Then, to determine the pH at the column outlet, we do place the column in the system, but we move the point of addition of the indicator dyes to a point between the column outlet and the detector. Again, the dyes never go through the column itself, as shown in Figure 1b.
Review from Last Time
In last month’s installment of “LC Troubleshooting,” we demonstrated the effect of pH mismatch between the mobile phase and injected sample on chromatograms obtained for the ionogenic analyte benzoic acid, and the control molecule benzyl alcohol (5). The mobile phase was buffered at pH 3.2, and samples buffered at pH 7.0 were injected. We varied the concentrations of both the mobile phase buffer (1, 15, and 100 mM phosphate) and the sample buffer (1, 15, and 100 mM phosphate) and injected volumes ranging from 1 to 100 µL to get a sense for the effect of the buffer mismatch over a wide range of conditions. As one would expect, the overall trend is that injecting smaller volumes of sample in cases where the mismatch in buffer concentration is small or favors the mobile phase (injecting a sample at 1 mM into mobile phase at 100 mM; see Figure 2 below) produces the best results.
Figure 2: Comparison of peak shapes for benzoic acid obtained with different injection volumes (colors), mobile-phase buffer of 100 mM phosphate at pH 3.2, and sample buffers of 1 or 100 mM phosphate at pH 7.0. Chromatographic conditions: Column, 50 mm x 4.6-mm i.d. SB C18; Flow rate, 2 mL/min.; Temperature, 40 °C; Mobile phase, 23:77 acetonitrile:buffer. Samples contained 13% acetonitrile, 87% buffer, and benzoic acid at a concentration of 20.7 µg/mL in each sample. For details on buffer preparation, see reference (6).
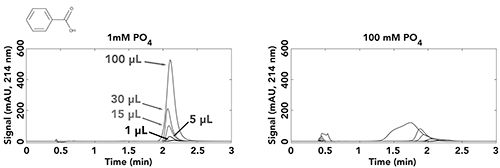
However, the results showed that if the injection volume is large (100 µL), even injecting a weakly buffered sample (1 mM, pH 7) into a moderately buffered mobile phase (15 mM, pH 3.2) can lead to poor results. Figure 2 shows a subset of these results for the case where the mobile phase is buffered with 100 mM phosphate buffer at pH 3.2, and the sample is buffered with either 1 or 100 mM phosphate at pH 7.0. Here we see that injecting the 1 mM sample yields good results even up to an injection volume of 100 µL, though this peak is slightly broader than the peaks for the smaller injection volumes (note that this broadening is not due to the injection volume; see Figure 2a from ref. [5]). However, when the sample buffer concentration is increased to 100 mM there is already a significant effect of the sample buffer on retention of benzoic acid at 15 µL, and by the time we get to 100 µL the peak shape is awful and some of the analyte is even eluted at the dead time. Now, we have the opportunity to visualize the pH changes that are actually happening in the column using the in situ pH determination approach.
In Situ Determinations of pH Under These Conditions
The results of our in situ pH determinations under the conditions shown in Figure 2 are shown in Figure 3. In each graph we have pH plotted vs. time (we refer to them as “pHgrams”), which shows the local pH of the mobile phase as it moves through the detector. The top two sets of pHgrams were obtained with no column installed (see Figure 1a), and thus effectively show us the pH of the mobile phase after the sample has been injected, but before it enters the column. The bottom two sets were obtained after the column, and thus show us the pH at the column outlet. In each case, the mobile phase was buffered at pH 3.2 with 100 mM phosphate. The left two sets of pHgrams were obtained with samples buffered at pH 7.0 with 1 mM phosphate, and the right two sets with 100 mM phosphate.
Figure 3: Four “pHgrams” at the column inlet (a) at 1 mM PO4 , and (c) at 100 mM PO4; and outlet (b) at 1 mM PO4, and (d) at 100 mM PO4. These pHgrams were obtained under the conditions described in Figure 2, using the setup shown in Figure 1. Line colors indicate injection volumes as shown in Figure 2. In this case, construction of the hue vs. pH calibration function was done using different ratios of 100 mM phosphate buffers at pH 2.5 and 7.5, all containing 23% acetonitrile as in the mobile phases used in Figure 2. The pH values were calculated assuming completely aqueous solutions. We realize that these are not the actual pH values in the acetonitrile buffer solutions, but the differences in pH due to the addition of acetonitrile do not affect the overall conclusions drawn from the interpretation of the pHgrams shown here.
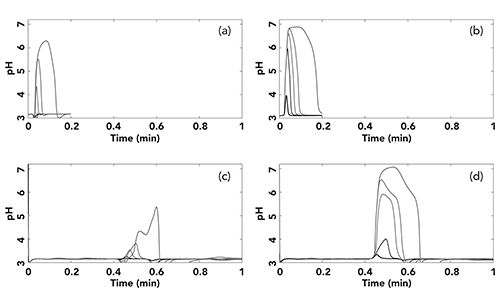
There are several interesting aspects of this set of pHgrams. First, Figure 3a shows us that even the weakly buffered sample of 1 mM phosphate produces a mobile phase zone that persists above pH 5 all the way to the column inlet when 30 or 100 µL of sample are injected. When the sample buffer concentration is increased to 100 mM, even the 5 µL sample produces a zone of pH 6 that persists at the column inlet. Moving on to the column outlet, we see that in general the maximum pH of the sample buffer zone as it exits the column is lower than the maximum pH of the zone entering the column. The most obvious explanation for this change is that the sample buffer band broadens due to dispersion in the column. This results in mixing and neutralization of the sample buffer with the mobile phase buffer. When the injected sample is 1 mM phosphate, this dispersion and neutralization results in a zone exiting the column that is always below pH 4, except for the 100 µL injection, where there is a small part of the zone that persists above pH 4. Because the pKa of benzoic acid is about 4.2, this reduction in the pH of the sample buffer zone travelling the column is consistent with the observation in Figure 2 that good peak shapes are observed even with injection volumes up to 100 µL. However, when the sample is buffered with 100 mM phosphate, the 15, 30, and 100 µL injection volumes yield large zones above pH 5 that persist all of the way through the column and to the detector. As one would expect, this zone of buffer above the pKa of benzoic acid results in a significant decrease in retention time and increasing in peak tailing (15 and 30 µL) and in the worst case a highly distorted and split peak (100 µL).
Closing Thoughts
Mobile-phase pH can have a significant effect on retention and peak shape for ionogenic analytes in several modes of liquid chromatography, and can be used to great effect for optimizing separations during method development. However, the effects of buffer type, concentration, and pH can be complex and lead to unexpected results. In this installment of “LC Troubleshooting,” we have used in situ determinations of mobile-phase pH to rationalize the effects of mismatch between the pH and concentration of mobile phase and sample buffers used in reversed-phase LC. We see that the in situ pH determination approach enables visualization of pH changes that happen inside the column, which are consistent with the behavior we observe for ionogenic analytes such as benzoic acid. Continued study of these effects will lead to a more complete understanding of all the variables in play when pH plays a role in retention, which in turn will lead to more efficient method development and more robust and effective LC methods.
References
- G.W. Tindall, LCGC North Am.20(11), 1028–1032 (2002)
- G.W. Tindall, LCGC North Am.20(12), 1114–1118 (2002).
- G.W. Tindall, LCGC North Am.21(1), 28–32 (2003).
- D.R. Stoll and D. Makey, LCGC North Am.37(7), 444–449 (2019).
- T.J. Lauer and D. R. Stoll, LCGC North Am.38(1), 10–15 (2020).
- G. Leme, B. Madigan, J. Eikens, D.C. Harmes, D. Richardson, P. Carr, and D. Stoll, Anal. Meth. 11 381–386 (2019). https://doi.org/10.1039/c8ay02496k

Thomas J. Lauer is currently a student researcher working in Dwight Stoll’s laboratory at Gustavus Adolphus College in St. Peter, Minnesota.

Dwight R. Stoll is the editor of “LC Troubleshooting.” Stoll is a professor and co-chair of chemistry at Gustavus Adolphus College in St. Peter, Minnesota. His primary research focus is the development of 2D-LC for both targeted and untargeted analyses. He has authored or coauthored more than 60 peer-reviewed publications and four book chapters in separation science and more than 100 conference presentations. He is also a member of LCGC’s editorial advisory board. Direct correspondence to: LCGCedit@mmhgroup.com

Troubleshooting Everywhere! An Assortment of Topics from Pittcon 2025
April 5th 2025In this installment of “LC Troubleshooting,” Dwight Stoll touches on highlights from Pittcon 2025 talks, as well as troubleshooting advice distilled from a lifetime of work in separation science by LCGC Award winner Christopher Pohl.
Mobile Phase Buffers in Liquid Chromatography: A Review of Essential Ideas
December 11th 2024In this installment of "LC Troubleshooting," Dwight Stoll discusses several essential principles related to when and why buffers are important, as well as practical factors, such as commonly used buffering agents, that are recommended for use with different types of detectors.
In-Loop Analyte Degradation in Two-Dimensional Liquid Chromatography: Example and Solutions
September 13th 2024In this installment of “LC Troubleshooting,” we describe an artifact that can arise because of compound degradation during the transfer of fractions of the first dimension (1D) column effluent to the 2D separation.





![[1].jpg [1].jpg](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2F0vv8moc6%2Fchroma%2Ff970d09fd9a78d8d758b609bc5555ac0efcf1f2b-1000x228.jpg%3Ffit%3Dcrop%26auto%3Dformat&w=3840&q=75)