Does the Position of Wool in the Inlet Matter?
Wool in the liner improves chromatographic performance by enhancing vaporization of high molecular weight compounds, promoting sample mixing, reducing backflash and trapping non-volatile matrix. We have received reports where the use of wool in the liner has resulted in poor reproducibility and low response for semi-volatile compounds. This study will replicate those conditions and evaluate the impact of wool position in the liner using different split ratios and splitless conditions.
There is a significant body of work detailing the optimization of gas chromatography (GC) inlets using splitless and split modes with a variety of liner configurations with and without wool. Linx Waclaski conducted a study evaluating four splitless liners for recoveries of hydrocarbons from C8 to C40. The single taper and straight liner showed a decrease in recovery of hydrocarbons (>C24) when compared to a single taper with wool and a double taper cyclo-gooseneck liner. The single taper with wool was found to be the one of the best liners since the wool enhanced the vaporization of heavier analytes (1). In another study comparing split liners using a 50:1 split ratio, the highest peak areas and the lowest relative standard deviations (RSDs) contained wool (2).
Work has been done using splitless injection techniques to verify that the amount of wool does not change peak responses and does not result in an increase in activity for most active compounds, such as pentachlorophenol and benzidine. Only when packed with three times the amount of specified wool was there was an observed decrease in response for 2,4-dinitrophenol, but not the other active analytes tested. Manufacturers deactivate the wool after it has been placed in the liner to prevent breaking the wool fibers. The un-deactivated wool fibers expose active silanols (3,4).
Over the years we have received calls from customers struggling with calibration curve linearity and reproducibility of standards when using either split or splitless straight 4 mm liners with wool as purchased from the manufacturer. The wool is placed about 25 mm into the liner, which is just below where a typical autosampler syringe needle would inject. Although the wool has not moved during shipping, one prevailing hypothesis is that the wool shifts in the liner while installed in the injection port, thereby changing the rate of vaporization. Replacing the septum while the injection port is pressurized may cause the wool to be pushed to the top of the liner, and if the needle pierces the wool the sample can be injected below the wool. In this scenario, the wool may not be effective at vaporizing the sample (Figure 1).

In this work, a 50:1 split, 5:1 split, and splitless conditions will be used to evaluate liners with wool in different positions. Wool in the center, wool at the bottom, wool at the top and no wool will be compared for responses and reproducibility as shown in Figure 1.
Experimental Design
The column used for this work was a 100% polydimethylsiloxane (PDMS), “1-type phase,” (Restek) 30 m, 0.25 mm internal diameter with a 0.25 μm film thickness installed into a GC–mass spectrometer (MS) with a flow of 1 mL/min. The split conditions of 5:1 and 50:1 used a GC oven program of 80 °C (hold 1 min) increased 20 °C per min to 300° (no hold), then 15 °C per min to 350 °C (hold 10 min). The splitless conditions used an oven program of 50 °C (hold 1.7 min) increased 20°C per min to 300 °C (no hold), then 15 °C per min to 350 °C (hold 8 min). The splitless hold time was set for 1.7 min. The MS scan rate was 5.6 scans per second and scanned from m/z 45 to 550 with a 2.6-min solvent delay. Compounds chosen were even-numbered alkanes ranging from C8 (n-octane) to C40 (n-tetracontane) in hexane. Concentrations were adjusted to maintain 10 ng on column for both split ratios, and splitless conditions were conducted at 50 ng on column. Split and splitless conditions were tested with wool in the center (as provided by manufacturer), no wool, wool at bottom, and wool at top pierced by autosampler syringe needle (Figure 1). Tests were performed five times and the position of the wool was inspected between liner changes. If the wool was found to have moved, the wool was repositioned and the liner was re-analyzed.
Results and Discussion
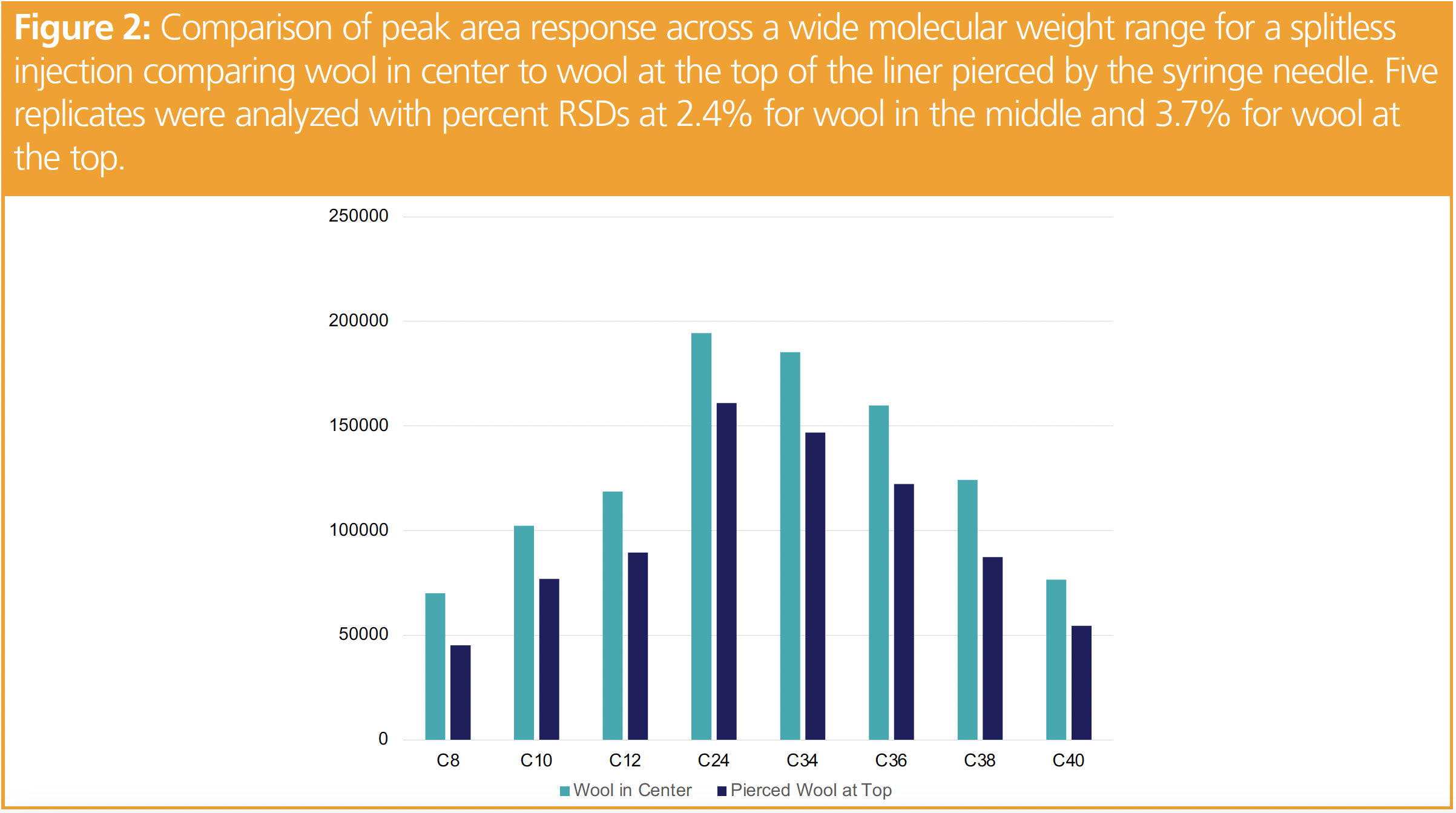
Splitless injections provide the best sensitivity since most of the sample (and solvent) is directed onto the column by closing the split line. Splitless injection techniques require careful optimization of the hold time, starting temperature, stationary phase choice and solvent. The slow flow rate in the liner can lead to solvent effects and degradation of active analytes. Since we were injecting hexane, our vapor cloud was calculated at 223 μL with an effective liner volume of 493 μL (5), which meant most of our sample was trapped during the hold time in the injection port and transferred to the column. Konrad Grob designed and built a glass injection port submersed in hot oil, where he injected perylene and was able to observe the compound with ultraviolet light. The results of his work demonstrated the Leidenfrost effect, where the solvent is held slightly above the surface of the inlet seal by a vapor cloud (6). This demonstrated the source of discrimination as the column is positioned above the inlet seal and less volatilized sample is lost. Figure 2 is a comparison of wool at the center compared to wool at the top with similar peak area responses. Not shown were the no wool, which was nearly identical to wool at the top, and wool at the bottom, which was nearly identical to wool in the middle. These results are similar to other published work on this topic (7).
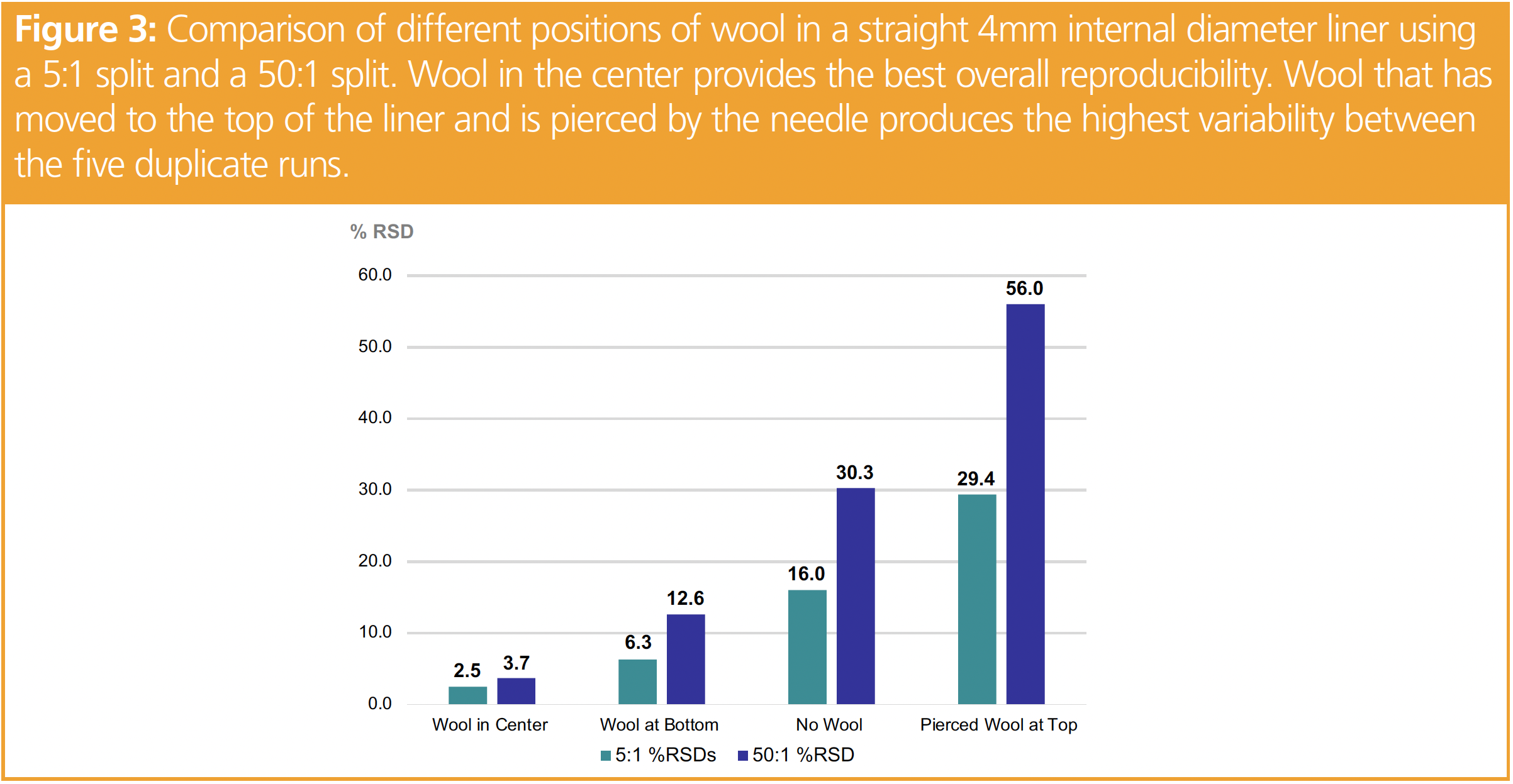
Split injections use high flow rates and reduce the amount of time the sample spends in the injection port. Wool or another mechanism is required to thoroughly vaporize the sample, which is then transferred to the column producing narrow peaks. Higher initial oven temperatures are possible because there is considerably less solvent transferred to the column (8). Figure 3 is a comparison of the different positions of wool in a straight 4 mm internal diameter liner using a 5:1 and a 50:1 split ratio. Not surprisingly, wool in the center provides the best overall reproducibility because the heater block and heat sensor are located in the middle of the injection port. With our setpoint of 250° C, the top and the bottom of the inlet can be 100 °C cooler (9). This accounts for higher RSDs, lower response and greater discrimination for higher molecular weight analytes for no wool, wool at the bottom and wool at the top. Wool that has moved to the top of the liner and is pierced by the needle produces the highest variability across the five duplicate analyses (Figure 3). Higher split ratios decrease the time the analytes spend in the injection port and therefore are not fully volatilized.
Replicating the customer’s issues could be done in split mode as long as two conditions were met: that wool was at the top of the liner and the autosampler needle penetrated the wool. In over half of the cases where the wool was placed at the top using an autosampler syringe with a cone-style tip, the wool was pushed back in place and the analysis mimicked the result for wool in the middle. For splitless analysis the differences were more subtle and could be related to other variables.
Conclusions
For split analysis, wool in the center of the liner provides the best vaporization of heavier analytes, resulting in the highest peak areas and the lowest standard deviations. Precision-style liners are recommended for split analysis since the plug of wool is held in place by baffles. For those using a straight liner with wool, always depressurize the inlet before maintenance and verify the wool has not moved. For splitless analysis, the wool at the bottom and the wool in the middle had similar recoveries. A single taper liner with wool at the bottom has been shown to provide excellent vaporization of the sample with decreased analyte breakdown (2).
We have found that the wool in a straight liner can move to the top of the liner following a septum change when the instrument has not been depressurized. If the needle pierces the wool, the sample can be injected below the wool, resulting in the highest RSDs measured for this study.
References
(1) Waclaski, L. Optimizing Splitless GC Injections. Column 2018, 14 (8), 11–15.
(2) Waclaski, L. Selecting a GC Inlet Liner. American Laboratory. CompareNetworks, Inc., 2016. https://www.americanlaboratory.com/914-Application-Notes/189474-Selecting-a-GC-Inlet-Liner/ (accessed 2024-05-14).
(3) Waclaski, L. Does the Amount of Wool in Prepacked Liners Matter?—Part I: Experimental Setup. ChromaBLOGraphy. Restek Corporation, 2020. https://www.restek.com/chromablography/does-the-amount-of-wool-in-prepacked-liners-matter-part-i-experimental-setup (accessed 2023-04-23).
(4) Waclaski, L. Does the Amount of Wool in Prepacked Liners Matter?—Part II: Results. ChromaBLOGraphy. Restek Corporation, 2020. https://www.restek.com/chromablography/does-the-amount-of-wool-in-prepacked-liners-matter-part-ii-results (accessed 2023-04-23).
(5) Solvent Expansion Calculator. Restek. Restek Corporation, 2024. https://www.restek.com/solvent-expansion-calculator (accessed 2024-05-14).
(6) Grob, K. Split and Splitless Injection in Capillary Gas Chromatography: With Some Remarks on PTV Injection, 3rd ed. Hüthig Buch Verlag Heidelberg, 1993. https://pdfs.semanticscholar.org/193a/e59f1d1e78a792bd383f8f45e5097f605892.pdf (accessed 2024-05-14).
(7) Waclaski, L. GC Inlet Liner Selection, Part I: Splitless Liner Selection. ChromaBLOGraphy. Restek Corporation, 2019. https://www.restek.com/chromablography/gc-inlet-liner-selection-part-i-splitless-liner-selection (accessed 2023-04-23).
(8) Cochran, J.; Rattray, C. Semivolatiles Analysis Using Split Injection. LCGC Europe—The Application Notebook 2016, 29 (9), 533–534.
(9) Grossman, S. It’s A Matter of Degrees, but Do Degrees Really Matter? Technical Literature Library. Restek Corporation, 2024. https://www.restek.com/articles/its-a-matter-of-degrees-but-do-degrees-really-matter (accessed 2024-05-14).
Chris English has managed a team of chemists in Restek’s innovations laboratory since 2004. Before taking the reins of the laboratory, he spent seven years as an environmental chemist and was critical to the development of Restek’s current line of volatile GC columns. Chris holds a BS in environmental science from Saint Michael’s College, USA. E-mail: chris.english@restek.com

Study Examines Impact of Zwitterionic Liquid Structures on Volatile Carboxylic Acid Separation in GC
March 28th 2025Iowa State University researchers evaluated imidazolium-based ZILs with sulfonate and triflimide anions to understand the influence of ZILs’ chemical structures on polar analyte separation.