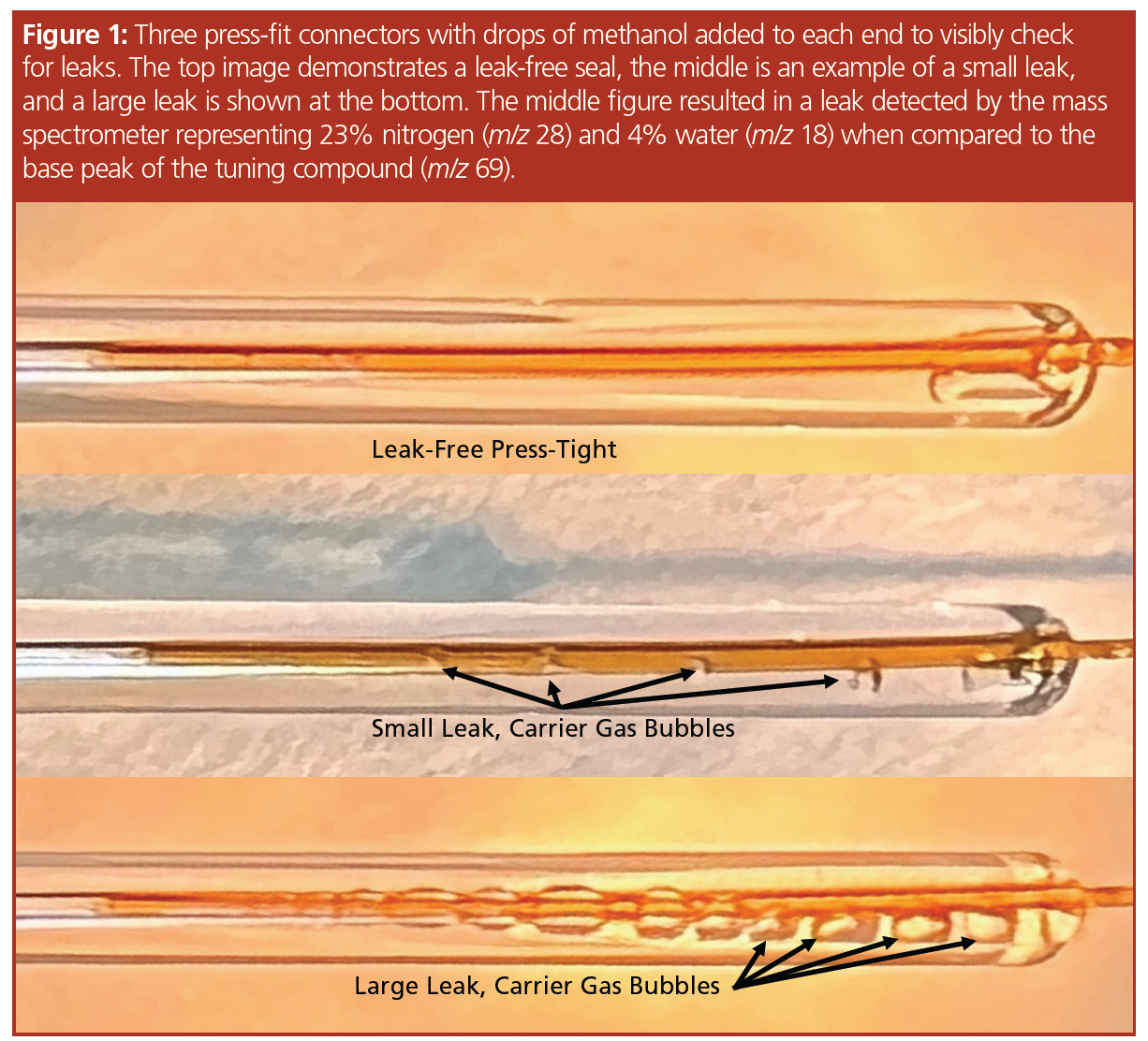
Do Small Leaks in Your Gas Chromatography System Matter?
Polysiloxanes (siloxanes) used for gas chromatography (GC) stationary phases are capable of withstanding repeated high-temperature cycles and injections of highly contaminated samples in a variety of solvents. Different thermal degradation pathways for siloxanes have been studied for decades, and in a dry, oxygen-free environment these materials can withstand temperatures of over 400 °C. Thermal oxidative degradation of siloxanes occurs through the bond scission of Si-0, and they subsequently form cyclic siloxanes. In addition, thermal hydrolysis also depolymerizes these stationary phases. By using a gas chromatograph mass spectrometer (GC–MS) system, the amount of water and oxygen reaching the detector relative to the tuning compound (perflurotribuytlamine [PFTBA]) can be measured. In this article, the different stationary phases will be evaluated, and their resistance to oxygen and water at a variety of temperatures will be tested.
In the last “Practical GC” instalment, the origins of cyclic siloxanes in a gas chromatography (GC) system were determined by analyzing septa bleed and column bleed (1). The depolymerization of these materials results in different distributions of cyclic siloxanes. In this article, air leaks in a GC system and their impact on GC stationary phases will be examined. In a recent review article, two different types of air leaks were described: large and small (2). This is a good place to start since large leaks inhibit the ability of the instrument to operate; for example, the injection port cannot maintain pressure, the mass spectrometer (MS) will not complete a tune, or sensitivity will be severely compromised. These leaks can be located using an electronic leak detector or pressure decay test. In another study, helium carrier gas was purchased with 1000 µL/L oxygen added to demonstrate the effects of a leak under controlled conditions. By using a three-way valve the analyses could be performed with and without a leak. Endrin and DDT (dichlorodiphenyltrichloroethane) breakdown was measured at nearly 40% and a standard containing 1 µg/mL could not reliably be detected. Baseline instability and high bleed were observed, and the column and liner were degraded. The conditions used were chosen to simulate 5% air entering the system (3). Our goal was to generate a small leak that was detectable by the GC–MS system but that would still allow the system to operate to include passing a perflurotribuytlamine (PFTBA) tune.
Experimental Design
Two different stationary phases were chosen: a “1-type” 100% polydimethylsiloxane (PDMS) and a “1701-type” composed of 14% cyanopropyl-phenyl with the remaining 86% as PDMS (Restek). Column dimensions for both columns were 30 m × 0.25 mm, 0.25‑µm and each column was installed into a GC–MS system (Agilent 7890 GC, 5975 MS) with a split/splitless inlet and a 100:1 split, constant flow 0.5 mL/min (1 psi @ 50 °C). The “1-type” GC program was 50 °C (hold 10 min), 10 °C per min to 200 °C (hold 10 min), 10 °C per min to 250 °C (hold 10 min), 10 °C per min to 300 °C (hold 10 min), 10 °C per min to 350 °C (hold 10 min), as shown in Figure 2. The “1701‑type” GC program was 50 °C (hold 10 min), 10 °C per min to 200 °C (hold 10 min), 10 °C per min to 250 °C (hold 10 min), 10 °C per min to 280 °C (hold 10 min). Both columns were operated to their maximum programmable temperature limits with and without leaks. A 1 m × 0.25 mm internal diameter guard column was installed in the split/splitless inlet connected to the analytical column using a press-fit connector. Connections were made without a leak and with a small leak, where the small leak was determined using the mass spectrometer to scan for m/z 69 (PFTBA base peak) and compare the abundance to air (nitrogen m/z 28) and water (m/z 18). To accomplish this, a press-fit connector was used between the guard column and the analytical column. Drops of methanol were added to the connection and a leak could be observed as bubbles exiting the connector (Figure 1). Nitrogen (m/z 28) and water (m/z 18) were compared to PFTBA’s base peak of m/z 69 to determine air and water relative to the abundance of the tuning compound and reported as percentages. To further verify that a leak was present, a leak detector and the use of methanol added dropwise to the connector was performed (Figure 1). A 1000 µg/mL pesticide standard of disulfoton, o,o,o-triethyl phosphorothioate, dimethoate, sulfotepp, methyl parathion, famphur, phorate, zinophos, and ethyl parathion dissolved in methylene chloride was used for this testing. The compounds have boiling points of between 200 °C and 400 °C. The MS scan rate was m/z 35 to 550 without a solvent delay.
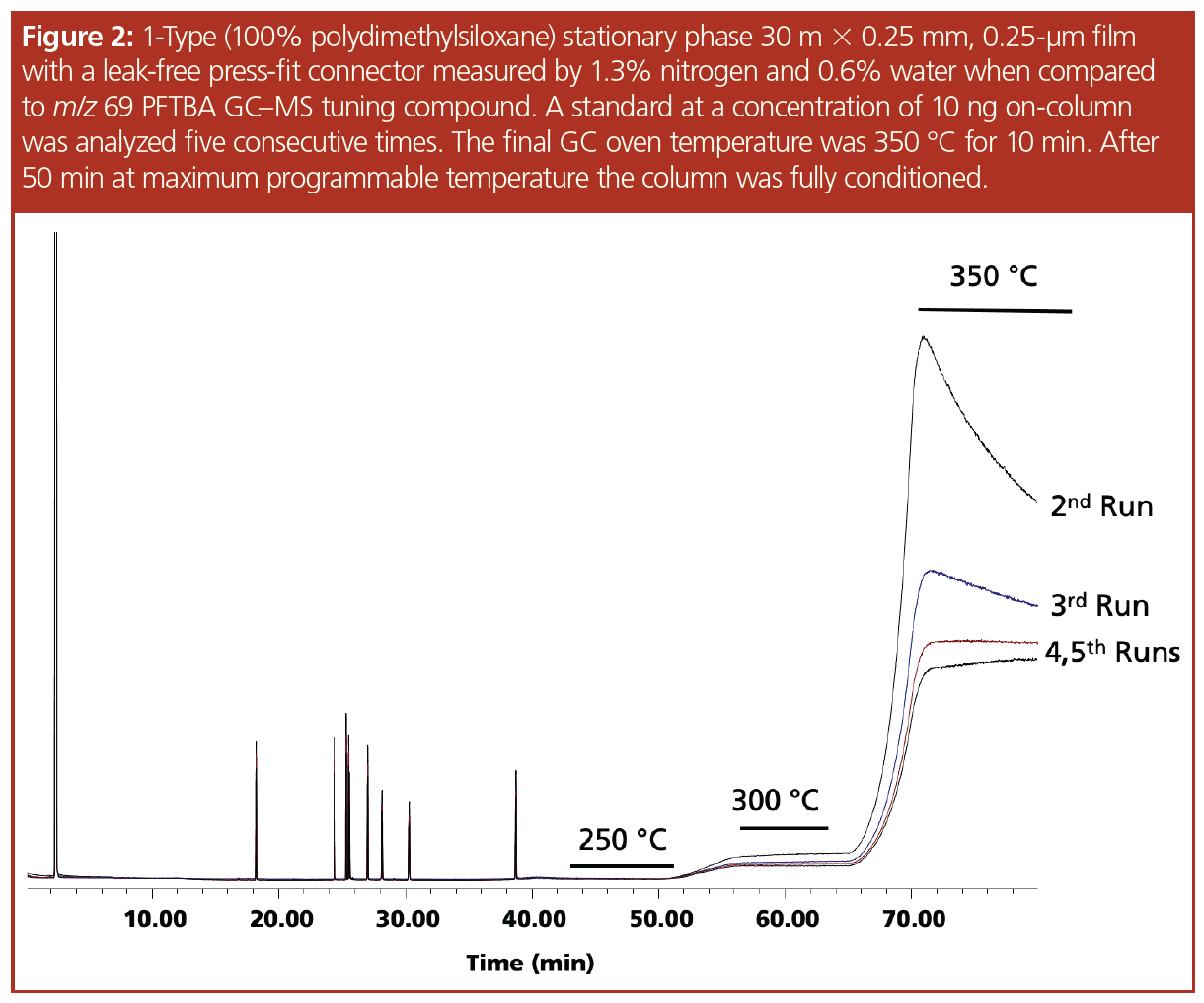
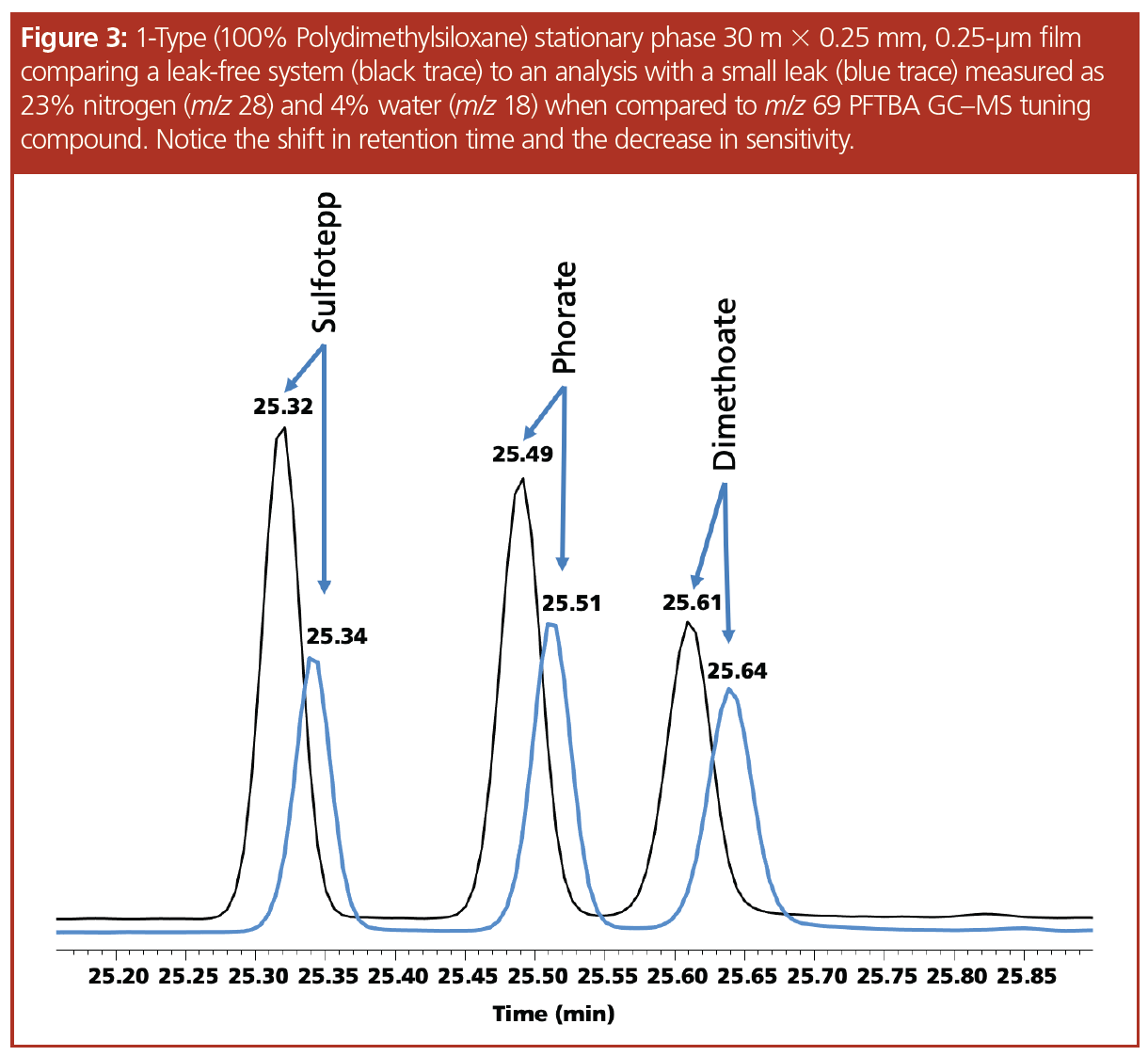
Discussion
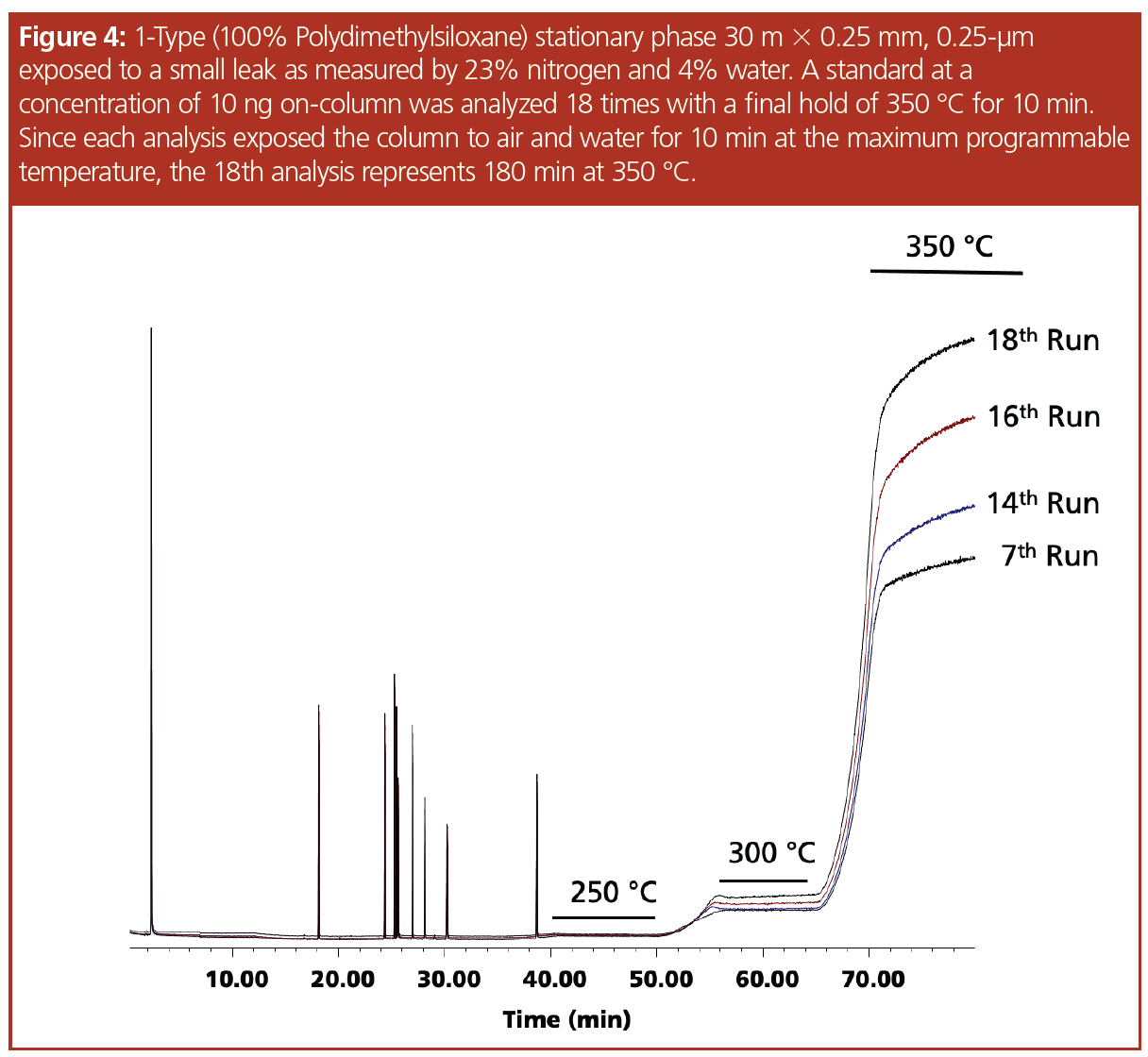
The “1-type” column used is stable to 350 °C, with variants of this phase with higher levels of cross-linking being able to withstand temperatures of 450 °C (4) using carrier gas free of oxygen and water (5). The column was installed and the system was determined to be leak-free using the mass spectrometer, a drop of methanol added to the connector (Figure 1), and a leak detector. An air and water check was performed indicating nitrogen at 1.3% and water at 0.6%. The pesticide standard was analyzed five consecutive times and the results of the runs were overlaid, indicating the column was properly conditioned—which was evident by the drop in the bleed profile and the stabilization of the baseline at the maximum programmable temperature (Figure 2). The connector was loosened and after many attempts a small leak was created, which was measured as 23% nitrogen and 4% water. The leak caused a slight change in retention time and a significant decrease in sensitivity (Figure 3). The standard was analyzed 18 times with a concentration of 10 ng on‑column to serve as a comparison to the bleed profile. Since each analysis exposed the column to the air and water leak for 10 min at the maximum programmable temperature, the 18th analysis represented 180 min at 350 °C. In Figure 4, several representative chromatograms are overlaid, showing a steady increase in bleed, as well as an increase in the slope of the bleed, indicating damage to the column. The “1701-type” column was installed; however, a consistent leak could not be maintained and while at the maximum programmable temperature of 280 °C, the column came out of the connector and therefore an accurate bleed profile could not be obtained. Even though the column was exposed to a large leak at the maximum programmable temperature, repeated conditioning decreased the baseline bleed. Analytes eluted on average 0.15 min earlier, indicating phase loss.
Conclusions
Previous studies have evaluated larger leaks in the system and found significant loss in sensitivity, analyte breakdown, high electron multiplier voltages, and frequent filament changes, as well as source cleaning (2,3). The goal of this article was to evaluate the impact of a small leak in the system that could be measured by the mass spectrometer but would allow the system to operate to include a passing tune. Introducing a small leak measured as 23% nitrogen and 4% water caused a retention time shift—a dramatic decrease in sensitivity and measurable increase in bleed. This further strengthens the case for proper gas management and illustrates that even trace amounts of air and water will adversely impact GC analysis, especially at higher temperatures.
Acknowledgements
Special thanks to Jaap de Zeeuw and Erica Pack (Restek) for their technical advice and review.
References
- C. English, The Column 18(6), 10–15 (2022).
- Agilent Technologies, Best Practice for Identifying Leaks in GC and GC/MS Systems (Technical Overview, Publication 5991-3899EN, 2014).
- K. Lynam, Impact of Air Leaks on the Productivity of GC and GC/MS Systems (Agilent Technologies, Application Note, Publication 5991-4110EN, 2014).
- C. Poole, Ed., Gas Chromatography (Elsevier, 2nd Ed., Amsterdam, The Netherlands, 2020), pp. 21, section 1.4.5.
- G. Camino, S.M. Lomakin, and M. Lazzari, Polymer. 42, 2395–2402 (2001).
Chris English has managed a team of chemists in Restek’s innovations laboratory since 2004. Before taking the reins of the laboratory, he spent seven years as an environmental chemist and was critical to the development of Restek’s current line of volatile GC columns. Prior to joining Restek, he operated a variety of gas chromatographic detectors conducting method development and sample analysis. Chris holds a B.S. in environmental science from Saint Michael’s College, USA.
E-mail: chris.english@restek.com
Website: www.restek.com

Study Examines Impact of Zwitterionic Liquid Structures on Volatile Carboxylic Acid Separation in GC
March 28th 2025Iowa State University researchers evaluated imidazolium-based ZILs with sulfonate and triflimide anions to understand the influence of ZILs’ chemical structures on polar analyte separation.