Determining Health Hazards: A Look into the EPA Practice
LCGC North America
The matter of perfluorinated compounds (PFCs) came to focus at the United States Environmental Protection Agency (EPA) some eight years ago.
This column traditionally has focused on practical aspects of how mass spectrometers function and has explored related topics like spectral interpretation. Yet, as the instruments and our practice with them evolve, we occasionally find important topics in which mass spectrometry (MS) plays a central role. PFCs are such a topic, and it warrants closer scrutiny.

Michael P. Balogh
As part of the Office of Research and Development National Exposure Research Laboratory (ORD/NERL), the Perfluorinated Compounds group develops analytical methods that reduce the uncertainty associated with human exposure to pollutants. Along with the health effects laboratory, which investigates traditional toxicology, the group investigates such issues as how people are exposed to pollutants and seeks to establish how to reduce that exposure. At CoSMoS in July 2007, Mark Strynar, a physical scientist in the Methods Development and Application Branch (MDAB) of the Human Exposure and Atmospheric Sciences Division (HEASD), discussed his group's efforts.
As a class, PFCs sprang to the forefront of issues at the EPA about eight years ago. These fully fluorinated compounds demonstrate qualities that make them useful for industrial and consumer product applications. At the molecular level, they possess a particularly stable carbon–fluorine bond, a bond comparable in its strength to carbon–carbon, carbon–nitrogen, carbon–oxygen, or carbon–chlorine bonds, all of which are very strong (Figure 1). PFCs resist atmospheric and biological degradation, which largely accounts for their commercial desirability. Nevertheless, they have attracted the interest of the EPA because they could prove to be bioaccumulative and toxic. In particular, the Organization for Economic and Cooperative Development (OECD) hazard assessment of PFOS reported carcinogenicity in lab animals and the Science Advisory Board to the EPA recently classified PFOA as "a likely human carcinogen."
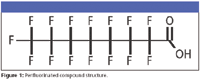
Figure 1
PFCs (Table I) are manufactured in various ways. The electrochemical fluorination process, no longer in use in the US for PFOS based chemistry, was one way. It employed a straight chain alkane in the presence of hydrogen fluoride (HF) and electricity. This fairly low-yield process led to not only even and odd numbered chains. but also to branched isomers, a fairly complex mixture of components. Until 2000, 3M (St. Paul, Minnesota) used this process to make the chemical backbone for Scotchgard, a coating for materials such as paper, upholstery, and carpet that makes such items impervious to damage from liquid spills.
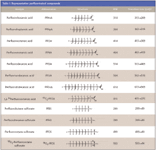
Table I: Representative perfluorinated compounds
Another PFC manufacturing method uses a telomerization process, where a telogen and a taxogen react to produce the telomer iodide. A benefit of this process is that it typically leads only to even-numbered chains.
Figure 2 illustrates a classic compound produced by telomerization, the 8:2 telomer alcohol (sometimes identified by the first full name, or the C-10 telomer). The 8:2 telomer alcohol is a perfluorinated chain with an ethanol group at its end. These species are quite volatile, and they do degrade somewhat (the ethanol group at the end can degrade, forming certain carboxylic acids). The most common perfluorinated structures detected are carboxylic acids (usually 5–11 carbon units) and sulfonic acids. The eight-carbon perfluorooctanoic acid (PFOA), industry's target compound, is the most common carboxylic acid. However, the reaction also yields a homologous series above and below PFOA. Similarly, the manufacture of perfluorosulfonates led not only to the target compound of perfluoroctanesulfonic acid (PFOS), but also to both shorter and longer perfluorinated sulfonic acids.
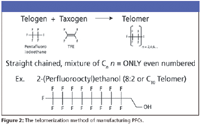
Figure 2
Though the PFOS-based chemistry was phased out in 2000, according to Strynar "With the increased use of telomer alcohols since the sulfonates were phased out, we find evidence in environmental samples that PFOS is just as present now as was reported then."
PFCs work well in certain applications because they are thermally stable and resist degradation. For instance, they are used to coat the surfaces of analytical equipment, which require resistance to acids, bases, and organic solvents (Table II). Very little fundamental chemical information has been tabulated, such as acid dissociation constants (pKa) and traditional octanol–water partitioning coefficient values (KOW ). The literature suggests this dearth of information is due to the surfactants' inability to partition into either octanol or water, yet they form a third phase. Without KOW values determining certain data like bioaccumulation factors is impossible.
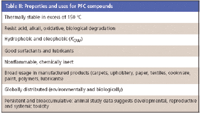
Table II: Properties and uses for PFC compounds
Pesticide manufacturers have used phosphinic and phosphonic acids as inert additives in some well-known formulations. Strynar says the compounds have been found in a number of locations, a fact that has generated interest in determining how much of it persists as a percentage of the amount sprayed.
So far, it is difficult to find standards for some of these compounds so "We are in the process of trying to make some, or clean up some, of the industrial compounds we have," says Strynar. "When I look through the literature of some of the companies that manufacture products made with PFCs, I realize that we've really seen only the tip of the iceberg. The list would include a lot of the things you and I have in our homes." Hence, the mission of the National Exposure Research Laboratory (NERL). "We want to know which, among the things you and I encounter every day, expose us most to these compounds. They are globally distributed; we find them in everything we examine — fish, air, water, soil — and the literature increasingly reflects that fact. The ubiquitous nature of these compounds is one of the things of great concern."
Bioaccumulation
How do PFCs accumulate in humans? Studies by the EPA's National Health & Environmental Effects Research Laboratory (NHEERL) find systemic toxicity, reproductive toxicity, immuno-toxicity, and developmental toxicity. For some of these compounds, the half-life in humans is relatively long at between four and nine years. "Anytime a toxin with a long half-life can interrupt human systems, it raises concern among people at the EPA," Strynar said.
The most recent exposure assessment work was done by 3M, which looked at its occupationally exposed workers (1). The levels of exposure of these workers to PFCs are extremely high compared to those of the average population. Following them years after they retired has allowed fairly accurate characterization of the PFC half-lives by comparison to what was found years earlier for these same people.
Most people, including those in the United States, who are exposed to PFCs show concentrations of about 30 ng/mL for PFOS and 5 ng/mL for PFOA (2). The exceptions are populations who don't come into contact with coated carpeting and upholstery and with microwavable food applications. Such populations evidence no PFCs in their bodies, but they comprise only a small minority of the world's people.
Water is one of the more extensively demonstrated linkages in the literature between exposure and body burden. Currently underway are EPA studies that examine how water effluents introduce industrial waste to the cycle. Efforts include investigations of environmental concentrations of PFCs in soil from all over the world. For instance, where products containing PFCs were sprayed as a preemergent herbicide, livestock feeds grown in contaminated soil can become a vector to distribute the PFCs globally, winding up in food products.
At its Washington Works facility on the Ohio River near Belpre, Ohio, DuPont makes PFOA. A municipal well field directly across the river serves the town of Little Hocking, Ohio. One study found in the blood of people who drink Little Hocking municipal water, about 374 ng/mL of PFOA, a far higher concentration than average (3). (For reference, the average person's blood reflects a concentration of 4–5 ng/mL.) People who didn't drink the municipal water but instead used private wells showed even higher concentrations in their blood, indicating movement of the compounds, which leech into the groundwater after crossing the river, ultimately appearing in soil and private wells.
Some states are a bit more proactive than Ohio. New Jersey, for example, imposed a drinking water standard of about 40 ng/L/ PFOA. West Virginia and Minnesota, two states in which 3M operates, determined they have problems. In the case of Minnesota, the state has imposed guidelines on 3M for concentrations of PFCs in drinking water.
The Analytical Realities
As Strynar points out, when trying to detect nanograms or picograms of a compound per sample, any perfluorinated compounds used to coat laboratory equipment becomes problematic. "We can obtain polypropylene tubes and vials free of perfluorinated compounds. But vendors sometimes switch to PFC-coated items without telling us. Then we experience intermittent contamination, which is most troubling: we use something for several months [without problem] and suddenly see contamination crop up in the method blanks."
One of the things Strynar's laboratory grapples with every day, much as we all do in analytical liquid chromatography (LC)–MS practice, is trying to find a blank matrix. The EPA prefers to perform matrix match standard curves and QC pools. However, according to Strynar, doing so can be difficult. "How do you find water, serum, soil, house dust, or fish — something that doesn't contain PFCs?" So much of the method development is devoted to finding something that is PFC-free.
Strynar uses a variety of equipment: an Agilent 5973 gas chromatography (GC)–MS system (Wilmington, Delaware) for telomer alcohols and an API/Sciex MS-MS system (PerkinElmer, Waltham, Massachusetts) with limits of detection to 0.5 pg on column, usually in negative electrospray ionization (ESI) mode. Atmospheric pressure chemical ionization (APCI) mode is unnecessary because the compounds of interest display sufficient sensitivity by ESI. A CRADA (cooperative research and development agreement) between EPA and Waters Corporation (Milford, Massachusetts) prescribes use of an ACQUITY UPLC system coupled with a Quattro Premier XE mass spectrometer for LC–MS-MS analysis with comparable subpicogram limits of detection.
Cape Fear
Examining water samples obtained throughout Japan, a study correlated what was found in water with what was found in humans, linking elevated serum concentrations of PFCs to people drinking contaminated water. Otherwise, besides this study of PFOS contamination of drinking water in the Tama River in Japan, there is little in the literature aside from some work in upstate New York based upon investigation of some isolated lakes, which for the most part was inconclusive (4).
The group at EPA in Research Triangle Park, North Carolina has worked to characterize the Cape Fear river basin (5) (Figure 3). Formed by the confluence of the Haw and Deep Rivers and ending in Wilmington, North Carolina, the Cape Fear river basin is the only large river basin fully contained within North Carolina. One of the rivers of the Cape Fear basin, the Little River, constitutes the northern border of the Fort Bragg and Pope Air Force Base military complex in Fayetteville, North Carolina.
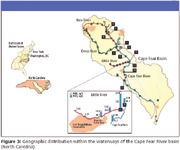
Figure 3
One of the applications of perfluorinated compounds, aqueous film-forming foam (AFFF), is used to extinguish chemical and petroleum fires. The training of personnel at military installations and municipal airports to fight jet fuel fires has been suggested to be associated with high concentrations of PFCs in the surrounding environment and, in this case, downriver from the Air Force base.
Samples were obtained in the field using a simple dip sampler — polyethylene bottles, a polyvinyl chloride (PVC) tube, and an extension rod. For depth samples, a Kemmerer sample device (an open stainless steel tube for taking depth samples in wells) was used.
A 0.5–1.0 L sample was filtered with a Whatman (Clifton, New Jersey) glass fiber filter. Solid-phase separations followed using various sorbents, as specified in the CRADA agreement with Waters. Elution was performed with 100% methanol, followed by concentration of the sample. Quantitation was achieved via multiple-reaction monitoring (MRM) MS. The chromatography used a perfluorinated, 100-cm high performance liquid chromatography (HPLC) column (Wako Pure Chemicals, Richmond, Virginia).
Strynar says, "We see some interferences at the beginning, and we can sometimes encounter difficulty separating the perfluorinated chemicals from humic material, which have similar chemical properties. The PFCs might stick with the humic material, or they might generate a "false positive" response that's not truly our compound. But for the most part, we can separate them adequately."
The instrument quantitation limit (IQL) as seen on the column with a 10-μL injection is 0.2 ng/L. For some work, the IQL can be as high as 1 ng/L, depending upon the limits expected for a particular compound.
PFOA and PFOS are the two most common PFCs, and they are the ones that have the greatest information on effects on health in laboratory animals. Other PFCs on the list currently are not associated with any health effects. The state of New Jersey mandates a drinking water guideline of 40 ng/L. Many of the samples taken in the Cape Fear water bodies exceed that guideline. Strynar's group sampled surface water in the basin's rivers. But Strynar says that just because PFCs exist in the river water doesn't mean they exist in the drinking water. No one has yet learned whether the compounds are removed during the drinking water purifying process. People in West Virginia and Ohio remove them with activated charcoal, but the majority of the drinking water facilities in the United States provide only chlorination and ozonation. No one has yet determined whether PFCs are removed in such systems. A value of 43 ng/L has been proposed as a guidance level for PFOS as protective of avian wildlife (6) and many, and many concentrations in the Cape Fear studies exceed that limit. The Little River, adjacent to the military installation, doesn't yield much PFOA. But it does yield a relatively large amount of PFOS, lending credence to the assertion of many that the AFFF is coming from Pope Air Force Base.
One of the things the EPA does when mapping these studies is, rather than showing the total concentration of PFCs, look at what they call the fingerprint. Strynar says that he can clearly see, at the confluence of the Haw River and the Deep River, a different chemical signature for which PFCs are present. If you go up the Haw River, you find mostly carboxylic acids. And if you go up Deep River, you find mostly sulfonates, mainly PFOS. Strynar is confirming those findings with work on fish caught and sediment samples taken. The work is still ongoing, but so far, it shows the same chemical signature in the fish and in the sediments.
The EPA had a program underway in Cincinnati called the Great Rivers Ecosystems Project. Samples were taken from the major rivers: fish, benthic organisms, water, sediment. Strynar, with access to archived fish samples, hoped to demonstrate whether PFCs could be measured in whole fish (5). Ecologically it matters little whether PFCs are found in the fillet (the portion we eat) or in the whole fish, because a bird or other predator eating the whole fish ultimately can impact humans as well. There's a "little bit of a bridge between demonstrating that the PFCs are there and their implications for human exposure and, but we are following up now."
To obtain the piscine samples, catfish, drum, bass, carp, carp sucker, red horse, shiners, and shad were sorted into these groups:
- piscivorous (fish-eating)
- nonpiscivorous
- pelagic (fish that roam between the top and bottom of the water column)
- benthic (bottom-dwelling species)
The groups were then divided into long-term and short-term fish, describing how long they live as adults. Short-term generally means that they live about three to four years in their adult stage.
To prepare the samples, a small amount of water was mixed with the fish, followed by an alkaline digestion with sodium hydroxide and methanol. The mixture was subject to centrifugation to separate the solids.
An aliquot of the mixture was passed through a Waters weak anion exchange cartridge. After conditioning and loading the column, an acetate buffer wash at pH 4 and then a 100% methanol wash were performed. The perfluorinated chemicals, retained on the column, remained there until an elution with ammonium hydroxide and methanol was performed.
The validated range was 0.2–4000 ng/g wet weight. The limit of quantitation (LOQ) for PFOS was set at 10 ng/g because the compound usually is present at higher amounts in the whole fish. The EPA performs a matrix spike standard curve with every quantitation. Says Strynar, "In some instances, some of that contamination from the background can crop up and [contribute] to our LOQ for the day. For instance, C6 was one that popped up and we isolated it to a batch of methanol. We've got Teflon seals on top of all the 4-L jugs, so when condensing a sample it's very likely you will see the contaminant."
The PFOS presence tends to be found anywhere between 50 and 70 ng/g of whole fish. According to Strynar, "With most other compounds, the C6–C12 acids and two sulfonates are no higher than 15, but most are down in the range 2–5 ng/g. When we compare long-term to short-term fish at the alpha 0.05 level, we note no statistical difference. When we compare piscivorous and nonpiscivorous fish at the alpha .05 level, we see significant differences, which indicate bioaccumulation from a larger, predatory fish eating a smaller one."
During his presentation at CoSMoS 2007, someone asked Strynar about the pharmacokinetics of PFCs in animal toxicology studies, whether there was any evidence of higher incidence of disease, and if so what diseases were related to exposure to PFCs?
We do work closely with a toxicologist at NHEERL, namely with PFOS and PFOA. In rodents, we find that for rats, male and female differ dramatically. In the case of PFOA, the female eliminates it, and the half-life is about three hours. The male takes about 14 days to eliminate PFOS.
In the CD1 mouse, we don't see any gender difference in the elimination of the PFOA. There is some evidence that organic transporters differ in male and female rats but not in male and female CD1 mice. For the PFOS we haven't seen any differences between the rat and the mouse."
No one can definitively find a linkage to human disease. [Some attempts] in the literature [have suggested links] with pancreatic cancer [the result of] an increase in incidence. The SAB [the EPA's Science Advisory Board] has found sufficient evidence to conclude that PFOA is a "likely human carcinogen." Yet so far they haven't seen any human health effects. We've not said [PFC contamination] is a problem because of [a specific] human disease. It's more its persistence [in the environment], its bioaccumulative nature, and the fact that it's everywhere that's been the concern."
Michael P. Balogh
"MS — The Practical Art" Editor Michael P. Balogh is principal scientist, LC–MS technology development, at Waters Corp. (Milford, Massachusetts); an adjunct professor and visiting scientist at Roger Williams University (Bristol, Rhode Island); and a member of LCGC's editorial advisory board.
Mark Strynar received the B.S. degree from the University of Rhode Island, Providence, the M.S. degree from Texas A&M University, College Station, Texas, and the Ph.D. degree from Pennsylvania State University, State College, Pennsylvania. He's currently employed by the EPA, in National Exposure Research Laboratory, which is part of the Office Of Research and Development in RTP. And his main task at the EPA is to develop analytical methods to reduce uncertainty in human exposure to pollutants.
References
(1) C. Fei, J.K. McLaughlin, R.E. Tarone, and J. Olsen, Env. Health Perspectives 11, 115 (2007).
(2) A.M. Calafat, Z. Kuklenyik, J.A. Reidy, S.P. Caudill, J.S. Tully, and L.L. Needham, Environ. Sci. Technol. 41(7), 2237–2242 (2007).
(3) E.A. Emmett, H. Zhang, S.F. Shofer, D. Freeman, V.N. Rodway, C. Desai, and L.M. Shaw, J. Occup Env. Med. 48(8), 759–770 (2006).
(4) K. Harada, N. Saito, N. K. Sasaki, K. Inoue, A. Koizumi, Bull. Environ. Contam. Toxicol. 71, 31–36 (2003).
(5) X. Ye, M.J. Strynar, S.F. Nakayama, J. Varns, L. Helfant, J. Lazorchak, A.B. Lindstrom, Environ. Pollut. (2008) article in press doi:10.1016/j.envpol.2008.03.014
(6) M.K. So, S. Taniyasu, N. Yamashita, J.P. Giesy, J. Zheng, Z., Fang, Environ Sci Technol. 38, 4056–4063 (2004).

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.












