Determination of PBDEs in River Water Utilizing Automated SPE in Conformance with EU Standards at IRSA
The Application Notebook
Polybrominated diphenyl ethers (PBDEs) are a worldwide contamination problem. Structurally similar to polychlorinated biphenyl (PCBs), these compounds are long-lived in the environment and can bioaccumulate throughout the food chain. The health hazards of these chemicals have attracted increasing scrutiny and, as such, a great deal of research and regulations have been implemented to manage and control them.
Brett Holmes and Michael Ebitson, Horizon Technology, Inc.
Polybrominated diphenyl ethers (PBDEs) are a worldwide contamination problem. Structurally similar to polychlorinated biphenyl (PCBs), these compounds are long-lived in the environment and can bioaccumulate throughout the food chain. The health hazards of these chemicals have attracted increasing scrutiny and, as such, a great deal of research and regulations have been implemented to manage and control them.
PBDEs have been used in a wide array of products, including building materials, electronics, furnishings, motor vehicles, airplanes, plastics, polyurethane foams, and textiles. Although many harmful PBDE congeners have been banned throughout the United States, many may still be present in equipment used today. As an international concern, the European Union has also banned multiple PBDE formulates, such as penta- and octa-BDE formulates, because of their persistence and ability to bioaccumulate within the environment.
The purpose of this investigation and method development was to establish a new procedure for the determination of PBDE congeners in aqueous samples ranging from the low ng/L (ppt) range, with a minimum of organic solvent consumption, while also conforming to the European Union standards.
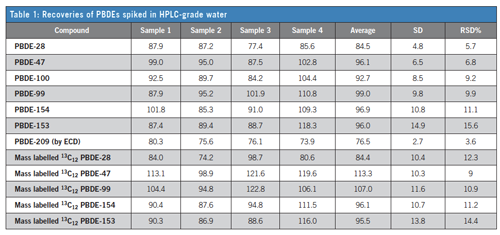
With assistance from the Italian Istituto di Ricerca Sulle Acque (IRSA), a fast and rigorous sample extraction and clean-up technique was developed that is selective for PBDEs. This was accomplished by optimizing a solid-phase extraction (SPE) disk method based on the Horizon Technology SPE-DEX® 4790 Automated Extractor System. The sample preparation step is an essential element of this method development, and as such, the advancement of an automated extraction and clean-up process has resulted in less solvent use, elimination of the solvent exchange step, reduced glassware use, faster extraction time, and more consistent and reproducible results. Table 1 shows results from spikes into reagent water extracted with SPE, showing excellent recovery and reproducibility. Additional work with river water is shown in the full note.
Reference
- Brett Holmes and Michael Ebitson, Application Note AN0551510_02, Determination of PBDEs in River Water Utilizing Automated SPE in Conformance with EU Standards at IRSA, available from www.horizontechinc.com

Horizon Technology, Inc.
16 Northwestern Drive, Salem, NH 03079 USA
Tel.: +1 603 893 3663 Fax: +1 603 893 4994
E-mail: spe@horizontechinc.com
Website: www.horizontechinc.com

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









