Determination of PAHs in Combustion-Related Samples via Multidimensional Chromatographic Methods
LCGC North America
Gas chromatography–mass spectrometry (GC–MS), reversed-phase LC with stop-flow fluorescence (FL), and constant energy synchronous fluorescence spectroscopy (CESFS) are explored to determine PAH isomers in three combustion-related standard reference materials.
Normal-phase liquid chromatography (LC) retention behavior was investigated for 239 polycyclic aromatic hydrocarbons (PAHs) on an aminopropyl (NH2) stationary phase. Retention behaviors were used to develop a normal-phase LC fractionation procedure for complex combustion-related samples prior to analysis with gas chromatography-mass spectrometry (GC–MS). Reversed-phase LC with stop-flow fluorescence (FL) and constant energy synchronous fluorescence spectroscopy (CESFS) capabilities were explored to determine PAH isomers of molecular mass (MM) 302 g/mol in normal-phase LC fractions. The combination of these analytical methods allowed for the determination of PAHs in three combustion-related standard reference materials (SRMs): SRM 1597a (coal tar), SRM 1991 (coal tar/petroleum extract), and SRM 1975 (diesel particulate extract).
Hugh V. Hayes, Walter B. Wilson, Lane C. Sander, Stephen A. Wise, and Andres D. Campiglia
Polycyclic aromatic hydrocarbons (PAHs) are a large class of environmental pollutants originating from natural and anthropogenic sources. PAHs with a molecular mass (MM) of 302 g/mol are of particular concern, due to their potential carcinogenic and mutagenic properties along with low biodegradability characteristics, namely, dibenzo[
a,l
]pyrene (DBalP), the most carcinogenic PAH tested to date. Unambiguous determination of DBalP and other MM 302 PAH isomers is imperative for accurate ecotoxicological assessment. PAHs have been identified in a variety of complex environmental matrices, such as coal tar, urban particular matter, and marine sediment, among others. The National Institute of Standards and Technology (NIST) provides an assortment of PAH-containing natural matrix standard reference materials (SRMs) such as SRM 1597a (coal tar), SRM 1991 (mix coal tar/petroleum extract), and SRM 1975 (diesel particulate extract). These SRMs are well-characterized, and often used for validating current and new analytical methodologies for PAHs.
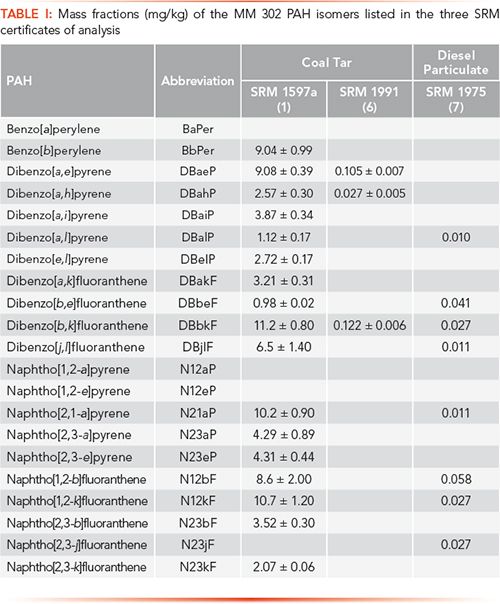
Methods for the separation and identification of PAHs typically employ reversed-phase liquid chromatography (LC) with fluorescence detection (FL) or gas chromatography–mass spectrometry (GC–MS). Excellent PAH separation has been observed using polymerically bonded octadecylsilane (C18) and 50% phenyl columns for reversed-phase LC (1) and GC–MS (2) conditions, respectively. However, structural isomers having similar retention times pose significant challenges, especially with GC, when these isomers have virtually identical mass fragmentation patterns. Normal-phase LC is another separation technique known for excellent separation of isomeric groups; however, low resolution of isomers groups with normal-phase LC makes accurate determination of specific PAHs a challenging task (3). Chemically bonded and polar functionalized stationary phases, such as aminopropyl (NH2), have been a practical approach for a class (number of aromatic carbons) fractionation prior to a secondary separation step (4). Table I summarizes the MM 302 PAH isomers identified in SRM 1597a (5), SRM 1991 (6), and SRM 1975 (7) via the traditional analytical methods described above. Herein, we explored the benefits of normal-phase LC fractionation as a sample clean-up step prior to further isomeric PAH separation and determination by both reversed-phase LC-FL and GC–MS (8–11). The combination of these analytical techniques provides a multidimensional approach to eliminating coelution and spectral interferences for PAH analyses.
Materials and Methods
Authentic PAH reference standards, SRM 1597a, SRM 1991, and SRM 1975 were obtained from multiple sources summarized elsewhere (8,9). Complete normal phase LC retention behavior was investigated for 239 PAHs, containing two to seven aromatic rings. Retention index values were determined for each PAH based on triplicate injections under normal-phase LC conditions. Separations were carried out on an NH2 analytical column, purchased from Waters, with the following characteristics: 25.0 cm length, 4.6 mm inner diameter, and 5 μm average particle diameters. An isocratic mobile phase of 98% n-hexane, 2% dichloromethane (DCM), and a 1.0 mL/min flow rate was utilized.
Normal-phase LC fractionation was completed using a Varian 9012 LC system (Agilent) with a Jasco UV-1570 Intelligent UV-vis detector. Normal-phase LC separations were carried out on an NH2 semipreparative column (Waters) with the following characteristics: 250 mm length, 10 mm inner diameter, and 5 µm average particle diameters. SRM 1597a was injected using a 250 µL sample loop with a mobile phase of 98% n-hexane, 2% dichloromethane (DCM), and a 4.0 mL/min flow rate. Fourteen fractions were collected from SRM 1597a, SRM 1991, and SRM 1975 using an in-house system over a 90 min separation interval, and evaporated with N2 to match injection volumes.
All SRM normal-phase LC fractions were analyzed directly using a 6890 series GC instrument (Agilent Technologies) using an HP 5973 quadrupole mass spectrometer with electron ionization (EI) (Agilent). GC separations were completed on a SLB-PAHms 50% phenyl stationary phase column with the following characteristics: 0.25-µm film thickness with a temperature maximum of 360 °C. PAH isomers were determined in each of the 14 fractions using selected-ion monitoring (SIM) mode. Peak identification in normal-phase LC fractions was determined by retention times and predominant mass ion peaks of authentic reference standards. The oven was temperature programmed to be isothermal at 100 °C for 1 min, 45 °C/min to 200 °C, 2 °C/min to 310 °C for 130 min, 45 °C/min to 325 °C, and isothermal at 325 °C for 60 min.
All reversed-phase LC-FL and reversed-phase LC-constant energy synchronous fluorescence spectroscopy (CESFS) analyses were carried out on an Ultimate 3000 Dionex HPLC system (Thermo Scientific) using an online degasser, a pump, and a UV-vis detector, along with a FL detector. Separations were performed on a Zorbax Eclipse polymeric PAH C18 column (Agilent) with the following characteristics: 250 mm length, 4.6 mm diameter, and a 5-µm average particle diameter. SRM 1597a fractions containing MM 302 PAH isomers (as determined by GC–MS) were separated using a mobile phase of 100% acetonitrile with a 1.5 mL/min flow rate. FL and CESFS spectral collection was obtained by using a stop-flow parameter in the instrumental software at the apex of each PAH chromatographic peak, which takes roughly 10–20 s per analyte. Quantitative reversed-phase PLC-CESFS measurements were performed using a linear regression model for synthetic mixtures of 13 targeted MM 302 PAH isomers prepared in toluene at concentration levels similar to Table I for SRM 1597a.
Results and Discussion
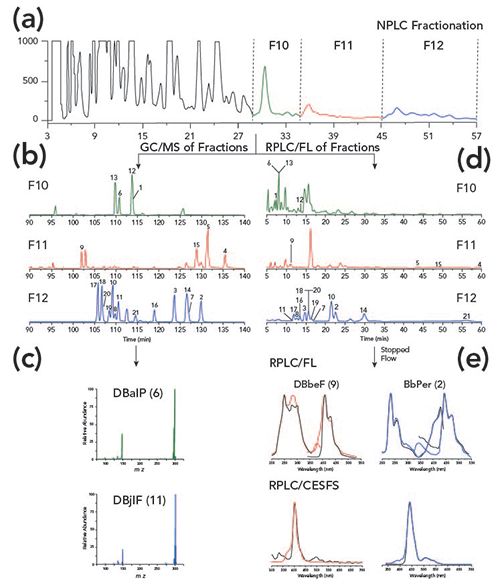
The schematic diagram for the analytical approach utilized in this work is detailed in Figure 1. SRMs were separated first by normal-phase LC and collected into 14 fractions. The normal-phase LC chromatogram for SRM 1597a with labeled fractions (F) is shown in Figure 1a. Each fraction was qualitatively analyzed by GC–MS in SIM mode to identify PAHs containing two to seven aromatic benzene rings (9). Quantitative GC–MS results after normal-phase LC fractionation have been published elsewhere by NIST (5–7), and was not a focus of this study. The MM 302 PAH isomers present in the three SRMs eluted in F10, F11, and F12. Figure 1b details the GC–MS chromatograms for F10, F11, and F12 for SRM 1597a, with the nearly identical mass spectra for DBalP and dibenzo[j,l]fluoranthene (DBjlF) shown in Figure 1c. After a comprehensive GC–MS evaluation of the isomers present in SRM 1597a, the three fractions containing MM 302 PAH isomers were then analyzed by reversed-phase LC with fluorescence detection (Figure 1d). The reversed-phase LC mobile phase was stopped at the apex of each chromatographic peak of interest for excitation/emission and synchronous emission spectral collection. Spectra collected in F10, F11, and F12 were compared with authentic reference standards for identification (Figure 1e). The reversed-phase LC-CESFS method was then quantitatively evaluated for thirteen MM 302 PAH isomers in SRM 1597a.
Figure 1: Schematic diagram for the separation and identification of MM 302 PAH isomers in SRM 1597a. The numbers correspond to the specific MM 302 PAHs in Table II and their molecular structures are published elsewhere (11). (a) Three fractions (F10, F11, and F12) were collected during the normal-phase LC separation of SRM 1597a. (b) All three fractions were qualitatively analyzed by GC–MS for the presence of MM 302 PAH isomers. (c) Mass spectra for DBalP and DBjlF. (d) All three fractions were further analyzed quantitatively by reversed-phase LC-FL. (e) Under stop-flow conditions, excitation/emission and synchronous emission spectra were collected during the reversed-phase LC-FL separation. Spectra for DBbeF and BbPer in SRM 1597a (colored line) were compared with authentic reference standards (black line). Chromatograms shown in (a) and (b) are published previously (9). Chromatograms and spectra shown in (d) and (e) are also published previously (11).
Normal-Phase LC
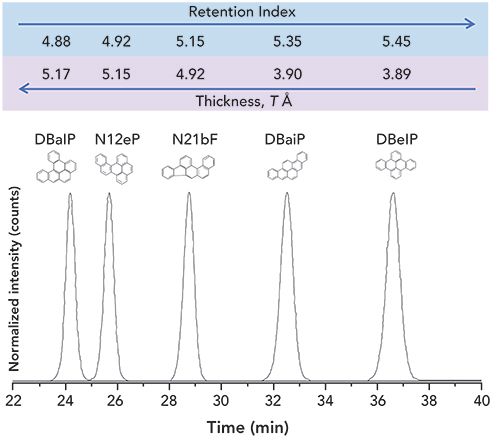
The isolation of isomeric PAH groups under normal-phase LC conditions has long been popularized by the efficiency of a semi-preparative NH2 column to separate PAHs based on the number of aromatic carbon atoms (8). The retention data for 239 PAHs are reported elsewhere (8). As seen in Figure 2, the retention of PAHs generally increases with increasing number of aromatic carbons. As the number of aromatic carbons increases for each isomeric group, the π-π interactions between the analyte and stationary phase strengthen, thus increasing the retention (8). Differences in retention behavior within each of the PAH isomer groups can mostly be attributed to differences in molecular thickness (non-planarity). Non-substituted PAHs with a thickness value of 3.90 are generally considered to be planar. As the molecular structure begins to deviate from planarity, PAH isomers are retained less on an NH2 column (8). As shown in Figure 3, the elution order of the five selected MM 302 PAH isomers is a direct representation of the decrease in thickness. DBalP is one of the least retained MM 302 PAH isomers due to the corkscrew nature of the molecular structure whereas dibenzo[e,l]pyrene (DBelP) is more retained with a planar shape. The planarity of the PAH also increases the overall π-π interactions with the NH2 stationary phase which increases column retention. Of the 25 studied MM 302 isomers, 6 of the 8 non-planar isomers elute before the remaining 17 planer isomers. The final two non-planar PAHs showed the largest affinity for the NH2 stationary phase due to the presence of bay-regions in their molecular structures. Similarly, bay-regions in other isomeric groups of different MM increased the affinity for the NH2 stationary phase (8). The normal-phase LC retention behavior was used to separate and isolate isomeric PAH groups in SRM 1597a, SRM 1991, and SRM 1975 into a total of 14 fractions over a 90 min time interval. It is important to note that the MM 302 PAH isomers were separated and collected in normal-phase LC F10, F11, and F12, as shown in Figure 1a.
Figure 2: Normal-phase LC retention behavior for various PAH isomeric groups detailed through chromatograms of selected PAHs of increasing number of aromatic carbons.
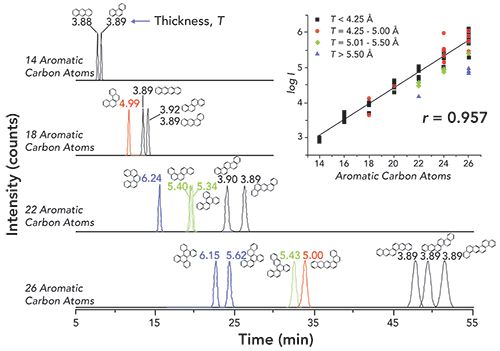
Figure 3: Normal-phase LC retention behavior for MM 302 PAH isomers. Retention on the NH2 column increases with increasing structural planarity. DBalP has an earlier retention time due to a larger thickness value while DBelP has a longer retention time with a smaller thickness value.
GC–MS
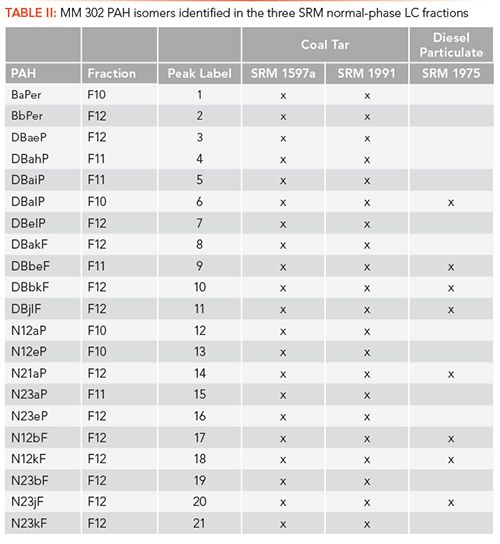
PAHs were determined in the normal-phase LC fractions of SRM 1597a, SRM 1991, and SRM 1975 using GC–MS programmed in selected-ion monitoring (SIM) mode. GC–MS analyses of MM 302 PAHs in F10, F11, and F12 of SRM 1597a are shown in Figure 1b. The number of MM 302 PAH isomers identified in F10, F11, and F12 were 4, 4, and 13, respectively, based on normal-phase LC retention behavior and GC retention times of authentic reference standards (9). The comparison of identified MM 302 PAH isomers in the three SRM certificates of analysis (COAs) and in the normal-phase LC fractions are listed in Table I and Table II, respectively. The analysis of SRM 1991 showed the greatest improvement by identifying 18 additional MM 302 PAHs when compared to those that are listed in the COA (6,9). The challenges with unambiguous identification by direct GC–MS analysis lie in the similarities in chromatographic behavior and molecular structures of these PAH isomers. One critical example includes the identification of DBalP, which is coeluted with DBjlF in the GC–MS separation of SRM 1597a without normal-phase LC fractionation. Mass spectra for these isomers are shown in Figure 1c, and have virtually identical mass fragmentation patterns making the unambiguous identification nearly impossible. Direct GC–MS analysis without fractionation increases the likelihood of misidentification of coeluted isomers, which limits the accuracy of the ecotoxicological assessment of the sample. However, DBalP and DBjlF are separated in normal-phase LC F10 and F12, respectively, due to the differences in structural non-planarity (9).
Reversed-Phase LC-FL and Reversed-Phase LC-CESFS
SRM 1597a was selected for the reversed-phase LC-FL investigations, based on the increased number of isomers identified by GC–MS. The use of reversed-phase LC-FL for compound identification is normally limited to comparisons of retention times of unknowns with authentic reference standards (10). The reversed-phase LC-FL chromatograms for F10, F11, and F12 are shown in Figure 1d. Unfortunately, accurate identification of PAHs based on retention times with coeluted concomitants may require additional chromatographic methods due to a lack of spectral information. Traditionally, reversed-phase LC fractions are collected, preconcentrated, and analyzed by spectrophotometric instrumentation for spectral identification or GC–MS (12). Here, stop-flow fluorescence detection was used to collect room-temperature fluorescence spectra of suspected PAH peaks (based on matching retention times) during reversed-phase LC separation of F10, F11, and F12. Spectral collection was obtained by stopping the mobile phase flow at the apex of each suspected chromatographic peak for immediate excitation and emission spectral collection. Total time for implementing stop-flow conditions and spectral collection was roughly 10–20 s, depending on the length of the analyte’s spectral profile. The spectra obtained from the fractions were compared to authentic reference PAH standards for identification. Reversed-phase LC-FL spectra obtained for DBbeF (F11) and BbPer (F12) in SRM 1597a fractions (colored) and authentic reference standards (black) are shown in Figure 1e. Full spectral profiles provide an additional layer of identification in complex matrices with minimal time of analysis. Of the 21 known MM 302 PAH isomers in SRM 1597a, 18 were identified based on reversed-phase LC retention times and spectral profiles with 8 having moderate to significant spectral interference (10).
While reversed-phase LC-FL with stop-flow capabilities proved beneficial for the analysis of complex environmental samples, the broad nature of room-temperature fluorescence invites significant spectral interference from coeluting concomitants. Here, we explore the benefits of CESFS in stop-flow conditions for the reversed-phase LC analysis of F10, F11, and F12. CESFS reduces spectral overlapping by simplifying excitation and emission spectral profiles into a single synchronous emission peak. Spectral simplification is achieved by scanning the excitation and emission monochromators simultaneously at a fixed wavelength distance (âλ) that is unique for each PAH isomer (11). Reversed-phase LC-CESFS spectra obtained in stop-flow conditions for DBbeF and BbPer in SRM 1597a fractions (colored) and authentic reference standards (black) are shown in Figure 1e. Of the 21 MM 302 PAH isomers known to be in SRM 1597a, 19 isomers were confirmed via reversed-phase LC-CESFS in F10, F11, and F12 with only three having spectral interferences (11).
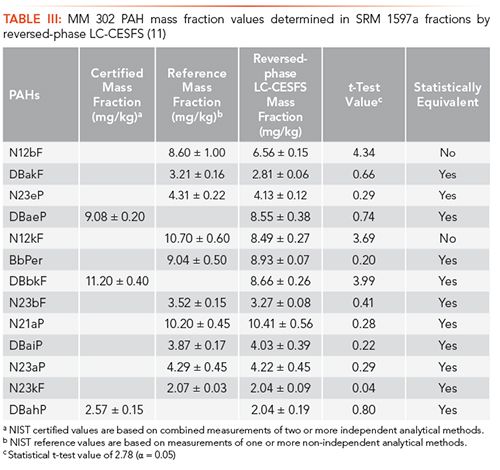
Since reversed-phase LC-CESFS provides excellent qualitative information with little to no spectral interferences, the quantitative capabilities were explored for methodology validation. Thirteen MM 302 PAH isomers were selected for quantitative evaluation of reversed-phase LC-CESFS by comparison to their assigned mass fraction values in the current COA for SRM 1597a. Signal intensities for synchronous emission peaks of the 13 PAH standards were used to construct external calibration curves to quantify these isomers in the SRM 1597a NPLC fractions. Of the 13 MM 302 PAH isomers, 10 showed statistically equivalent mass fraction values by both reversed-phase LC-FL and reversed-phase LC-CESFS when compared to the reported COA values (see Table III) (11). The three PAH isomers that did not provide statistically equivalent mass fraction values were lower than the reported COA values. It was concluded that the signal loss may be due to inner filter effects caused by coeluted interferences of unknown concomitants (11).
Conclusion
The results presented in this work showcase the multidimensionality of chromatography for the separation and determination of PAHs in complex matrices with minimal coelution and little to no spectral interferences. Normal-phase LC retention behavior was used to develop a normal-phase LC fractionation procedure coupled with GC–MS for the determination of over 200 PAHs in environmental samples. Analyzing individual normal-phase LC fractions by GC–MS significantly reduced the coelution of structural isomers having virtually identical mass fragmentation patterns in SRM 1597a, SRM 1991, and SRM 1975. In addition to GC–MS, reversed-phase LC with stop-flow fluorescence detection capabilities was also explored for the analysis of SRM 1597a normal-phase LC fractions to improve GC–MS misidentification due to coeluted structural isomers. Obtaining excitation and emission profiles for reversed-phase LC chromatographic peaks of interest added a new level of identification for MM 302 PAH isomers in SRM 1597a. Similarly, CESFS was utilized in stop-flow conditions to record synchronous emission profiles for MM 302 PAHs in SRM 1597a fractions with the goal of eliminating spectral interferences. To evaluate the accuracy of the reversed-phase LC-CESFS methodology, 13 MM 302 PAH isomers in the COA of SRM 1597a were investigated. The CESFS approach provided statistically equivalent mass fraction values for 10 of the 13 isomers showcasing the validity of this nondestructive methodology. The combination of normal-phase LC, GC–MS, and reversed-phase LC with collection of additional spectral features provides a multidimensional approach for the identification of PAH isomers in complex environmental samples.
Acknowledgments
This research was made possible by a grant from The Gulf of Mexico Research Initiative (Grant 23167-00).
Disclaimer
Certain commercial equipment or materials are identified in this paper to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
References
- L.C. Sander and S.A. Wise, LCGC North Am. 8, 378–390 (1990).
- F. Nalin, L.C. Sander, W.B. Wilson, and S.A. Wise, Anal. Bioanal. Chem. 410, 1123–1137 (2018).
- W.B. Wilson, H.V. Hayes, L.C. Sander, A.D. Campiglia, and S.A. Wise, Anal. Bioanal. Chem. 409, 5291–5305 (2017).
- S.A. Wise, S.N. Chesler, H.S. Hertz, L.R. Hilpert, and W.E. May, Anal. Chem. 49, 2306–2310 (1977).
- S.A. Wise, D.L. Poster, S.D. Leigh, C.A. Rimmer, S. Mossner, P. Schubert, L.C. Sander, and M.M. Schantz, Anal. Bioanal. Chem. 398, 717–728 (2010).
- Certificate of Analysis Standard Reference Material (SRM) 1991, Mix Coal Tar/Petroleum Extract in Methylene Chloride. National Institute of Standards and Technology, Gaithersburg, MD. https://www-s.nist.gov/srmors/view_cert.cfm?srm=1991
- Certificate of Analysis Standard Reference Material (SRM) 1975, Diesel Particulate Extract, National Institute of Standards and Technology, Gaithersburg, MD. https://www-s.nist.gov/srmors/view_cert.cfm?srm=1975
- W.B. Wilson, H.V. Hayes, L.C. Sander, A.D. Campiglia, and S.A. Wise, Anal. Bioanal. Chem. 409, 5291–5305 (2017).
- W.B. Wilson, H.V. Hayes, L.C. Sander, A.D. Campiglia, and S.A. Wise, Anal. Bioanal. Chem. 409, 5171–5183 (2017).
- H.V. Hayes, W.B. Wilson, L.C. Sander, S.A. Wise, and A.D. Campiglia, Anal. Meth. 10, 2661–2794 (2018).
- H.V. Hayes, W.B. Wilson, A.M. Santana, A.D. Campiglia, L.C. Sander, and S.A. Wise, Microchemical J. 149(9), 104601 (2019).
- W.B. Wilson, B. Alfarhani, A.T. Moore, C. Bis
Hugh V. Hayes and Andres D. Campiglia are with the Department of Chemistry at the University of Central Florida, in Orlando, Florida.
Hayes, Walter B. Wilson, Lane C. Sander, and Stephen A. Wise are with the Chemical Sciences Division of Material Measurement Laboratory at the National Institute of Standards and Technology, in Gaithersburg, Maryland. Direct correspondence to: hugh.hayes@nist.gov







In the present study, a gradient reversed-phase high-performance liquid chromatography (RP-HPLC) method has been designed and validated to quantify ornidazole (OZ) in the marketed formulation (oral gel) with the application of QbD.
A column with chemically modified column hardware showed improvements in analytical performance for siRNA compared to a conventional stainless-steel column.
In the present study, a gradient reversed-phase high-performance liquid chromatography (RP-HPLC) method has been designed and validated to quantify ornidazole (OZ) in the marketed formulation (oral gel) with the application of QbD.
A column with chemically modified column hardware showed improvements in analytical performance for siRNA compared to a conventional stainless-steel column.
2 Commerce Drive
Cranbury, NJ 08512

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)