Determination of Olive Oil Adulteration by Principle Component Analysis Using HPLC-Charged Aerosol Detection
Adulteration is a significant problem for the olive oil industry because of the product’s high value. With the demand for olive oil expected to rise significantly, this problem is set to increase. Reliable and accurate determinations of olive oil adulteration are therefore required to maintain product integrity. This article introduces a principle component analysis‑based approach that uses data obtained by high performance liquid chromatography (HPLC)-charged aerosol detection (CAD) to determine extra virgin olive oil adulteration by common vegetable oil adulterants. Using only sample dilution and analysis, the method could be used to assess olive oil quality relative to pomace oil.
Photo Credit: Marko Poplasen/Shutterstock.com

Khalil Divan, Thermo Fisher Scientific, Hemel Hempstead, Hertfordshire, UK
Adulteration is a significant problem for the olive oil industry because of the product’s high value. With the demand for olive oil expected to rise significantly, this problem is set to increase. Reliable and accurate determinations of olive oil adulteration are therefore required to maintain product integrity. This article introduces a principle component analysisâbased approach that uses data obtained by high performance liquid chromatography (HPLC)-charged aerosol detection (CAD) to determine extra virgin olive oil adulteration by common vegetable oil adulterants. Using only sample dilution and analysis, the method could be used to assess olive oil quality relative to pomace oil.
Adulteration of extra virgin olive oil (EVOO) with oils of lesser quality is a significant issue for the industry because of the product’s high market value. Commonly used adulterants (1,2) include lampanteâgrade olive oil (3) and other vegetable oils such as canola, avocado, palm, and sunflower oils (4,5). With future shortages of olive oil anticipated (6), and the associated increase in product value, it is likely that adulteration will become an escalating problem for the industry.
Producers therefore require reliable and accurate analytical techniques to characterize the chemical composition of their products and to confidently identify adulteration. Such analyses can also be useful for internal quality control purposes.
Given the complexity of the analysis required to verify the authenticity of olive oil, which has distinct chemical markers, sophisticated data management is necessary and an informatics solution comprised of a laboratory information management system (LIMS), a chromatography data system (CDS), a scientific data management system (SDMS) as well as a procedural electronic laboratory notebook (ELN) or laboratory execution system can offer a number of benefits around consistency of analysis and data integrity. Analyzing the data generated also requires care because not only is the concentration of individual components required, but also the relative concentrations to determine the authenticity of the product.
Principle component analysis (PCA) offers a powerful solution for characterization, quality control, and product brand protection. PCA is a statistical technique that analyzes the differences and similarities between samples, and has previously been used to quantify and differentiate adulterants in orange juices using high performance liquid chromatography (HPLC) and electrochemical array detectors (7).
In this article, a PCA-based approach that uses data obtained by HPLC-charged aerosol detection (CAD) to determine EVOO adulteration by three common vegetable oils-hazelnut oil, corn oil, and pomace oil-is presented. The results obtained by triglyceride analysis of whole oils, and those obtained by free fatty acid analysis of hydrolyzed oil samples, were compared to evaluate the best method for sample preparation and analysis.
This approach, carefully managed within an integrated informatics framework, could be used by manufacturers or contract laboratories as a potential automated method for the identification of olive oil adulteration. The compliance rules and instrument calibration checks offered by these electronic workflows ensure results are legitimate and legally defensible. Integration with the raw instrument data ensures that in the event of an audit or legal case, data can be followed from the analytical instrument through to the analyst’s conclusion.
Experimental
Materials: Mixed EVOO samples were prepared containing various proportions (1%, 5%, 10%, 25%, 50%, and 75% by mass) of corn oil (added to EVOO-1), and hazelnut oil and pomace oil adulterants (added to EVOO-2).
Sample Preparation: For EVOO samples that were hydrolyzed under base-catalyzed conditions, 50 μL aliquots were added to 4 mL of 3:2 2-propanol–water, followed by the addition of 1 mL of 5 M potassium hydroxide. Samples were heated in a water bath at 80 °C for 1 h. From the top layer (wet 2-propanol), a 500 μL aliquot was transferred to an HPLC vial and 25 μL of formic acid was added. Vials were capped and shaken. Unhydrolyzed olive oil samples were diluted 100 μL into 900 μL of 1:1 methanol–chloroform and analyzed directly.
HPLC System: An UltiMate 3000 system (Thermo Scientific Dionex) was used. Chromatograms were obtained and compiled using Chromeleon CDS v7.1 SR 1 software (Thermo Scientific).
Chromatographic Method (Hydrolyzed Samples): Analytical column: 2.1 × 250 mm, 2.6-μm Accucore (Thermo Scientific); mobile phase A: 900:100:3.6 methanol–mobile phase B–acetic acid; mobile phase B: 675:225:100:4 acetone–acetonitrile–tetrahydrofuran–acetic acid; flow rate: 0.4 mL/min; gradient conditions: -5.0 min 0%B, 0.0 min 0%B, 1.0 min 60%B, 13.0 min 7%B, 22.0 min 95%B, 24.0 min 95%B, 24.0 min 0%B, 27.0 min 0%B; column temperature: 50 °C; injection volume: 2 μL.
Chromatographic Method (Unhydrolyzed Samples): Analytical column: 3.0 × 100 mm, 2.6-μm Accucore C18 (Thermo Scientific); mobile phase A: acetonitrile; mobile phase B: 2-propanol; gradient conditions (flow rate): -6.0 min 0%B (1.0 mL/min), -0.2 min 0%B (1.2 mL/min), -0.1 min 0%B (0.8 mL/min), 0.0 min 0%B (0.8 mL/min), 0.2 min 0%B (1.2 mL/min), 2.0 min 10%B (1.2 mL/min), 25.0 min 40%B (1.2 mL/min), 30.0 min 60%B (1.0 mL/min), 30.0 min 0%B (1.0 mL/min); column temperature: 50 °C; injection volume: 5 μL.
Charged Aerosol Detection: Instrument: Corona ultra RS (Thermo Scientific Dionex); detector filter: 4; power function: 1.00, 1.20 (hydrolyzed, unhydrolyzed samples); data acquisition rate: 10 Hz; nebulizer temperature: 12 °C, ambient (hydrolyzed, unhydrolyzed samples).
CAD uses nebulization to create aerosol droplets of nonvolatile and semivolatile analytes. The mobile phase evaporates in the drying tube, leaving analyte particles, which become charged in the mixing chamber. The charge is then measured by a femtoampere-level electrometer, providing reproducible, nanogram-level detection limits. The detector does not need to ionize analytes and does not require analytes to possess a chromophore. This detection approach has greater sensitivity and precision than evaporative light scattering and is less expensive to operate than a mass spectrometer.
Results and Discussion
For omega free fatty acid analysis of hydrolyzed oils, aliquots of the mixed EVOO samples containing corn oil (EVOO-1) and a combination of hazelnut oil and pomace oil (EVOO-2) were hydrolyzed according to the above method and analyzed by HPLC using a C30 solid-core column. Chromatogram peaks were normalized to the stearic acid peak (Figure 1), integrated, and subjected to targeted PCA analysis using percent peak area values. The power function of the charged aerosol detector was used to eliminate bias of nonlinear correlation between composition and concentration (with a nonlinear correlation, peak areas would change depending on the amount of sample injected).
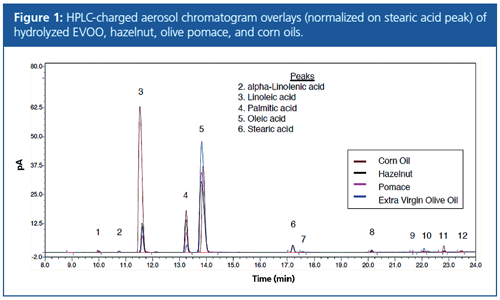
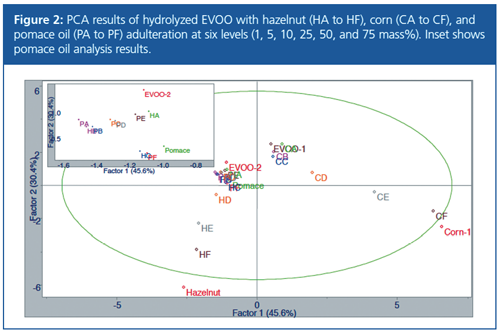
The results of the PCA (Figure 2) show that hazelnut and corn oil adulteration were clearly detected. However, adulteration with pomace oil was not as evident. As pomace oil is produced using heat and solvents to remove residual oils from the remains of previous olive pressings and extractions, pomace oil will have a similar profile to olive oil, and this was consistent with the results of the chromatograms and PCA.
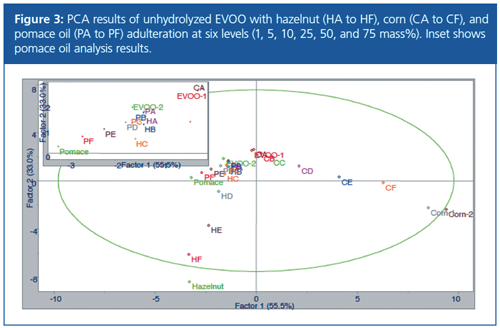
The same mixed oil and blend samples were diluted and analyzed directly using a solid core C18 column. HPLC chromatograms of the untreated samples were found to have significantly more peaks and more significant differences than the chromatograms of the hydrolyzed samples.
These triglyceride and diglyceride peaks were integrated and processed by targeted PCA. The results appeared to be better correlated between the original EVOO sample and the adulterated samples, with the adulteration of pomace oil particularly improved over the results using the hydrolyzed oil (Figure 3).
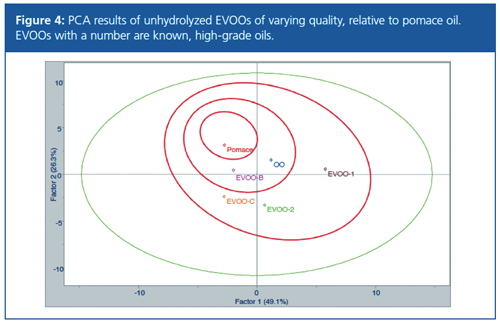
This analysis approach presents a possible measure of quality based on the distance of the sample from the pomace oil reference point. The procedure was used to determine the quality of known and unknown olive oil samples (Figure 4). Two known, high quality EVOOs (EVOO-1 and EVOO-2) were found to have the greatest difference from pomace oil. A lower quality olive oil (OO), extracted from the remains of olives after EVOO is obtained, and two lower quality EVOO samples (EVOO-B and EVOO-C), were found to lie between these extremes.
Conclusions
Using a PCA approach based on data obtained using HPLC-CAD, adulteration of EVOOs with common vegetable oil adulterants could readily be determined. The unhydrolyzed sample preparation approach was found to be more effective than the base-catalyzed hydrolysis approach, reducing the complexity of sample preparation. This method may be combined within an integrated informatics, comprising LIMS, SDMS, and ELN, to provide the olive oil industry with a simple, robust, and automated approach for assessing product purity.
References
- D. Marini, in Food Analysis by HPLC, L.M.L Nollet, (Marcel Dekker, New York, USA, 2000), pp. 231–232.
- R. Aparicio and R. Aparicio-Ruiz, J. Chromatogr. A881(1–2), 93–104 (2000).
- C. Higgins, Suspected Fraud Hits Major Israeli Supermarkets. Olive Oil Times, 2012. http://www.oliveoiltimes.com/olive-oil-business/africa-middle-east/oliveoil-fraud-supermarkets-israel/29281
- C. Cord, New Study Finds Some Foodservice Olive Oil “Not Fit for Consumption”. Olive Oil Times, 2012. http://www.oliveoiltimes.com/olive-oil-basics/somefoodservice-olive-oil-not-fit-for-consumption/28788
- J. Butler, Spanish Police Say Palm, Avocado, Sunflower Was Passed Off as Olive Oil. Olive Oil Times, 2012. http://www.oliveoiltimes.com/olive-oilbusiness/europe/operation-lucerna-olive-oil-fraud/24815
- I. Putinja, World Olive Oil Shortage Leads to Higher Prices. Olive Oil Times, 2015. https://www.oliveoiltimes.com/olive-oil-business/world-olive-oil-business/olive-oil-shortage-leads-to-higher-prices/48529
- P. Gamache et al., in Methods to Detect Adulteration of Fruit Juice Beverages, S. Nagy and R.L. Wade, Eds. (Agscience, Inc., Auburndale, Florida, USA, 1995) pp. 120–144.
Khalil Divan is the senior director of global marketing for the food and beverage market in chromatography and mass spectrometry at Thermo Fisher Scientific. He works closely with regulatory bodies, method validation associations, and key opinion leaders to address the needs of the food and beverage community.
E-mail:khalil.divan@thermofisher.comWebsite:www.thermofisher.com/uk/en/home/industrial/food-beverage/food-beverage-learning-center/food-analytical-testing-information/food-adulteration-analysis-information.html

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










