Tips & Tricks: New and Old Approaches to Handle Unknown dn/dc in GPC/SEC-LS
The intensity of the light scattering signal depends on concentration, molar mass, and the specific refractive index increment, dn/dc, of the sample. dn/dc therefore usually needs to be known to derive molar masses by gel permeation chromatography/size-exclusion chromatography-light scattering (GPC/SEC-LS). How to overcome the issue of unknown refractive index increments is outlined in this instalment of Tips & Tricks. In particular, a new approach to derive molar masses by GPC/SEC-LS that requires only the molar mass of a reference material, and not the dn/dc of the sample or of the reference material, is introduced.
Photo Credit: T.Sumaetho/Shutterstock.com

Wolfgang Radke, PSS Polymer Standards Service GmbH, Mainz, Germany
The intensity of the light scattering signal depends on concentration, molar mass, and the specific refractive index increment, dn/dc, of the sample. dn/dc therefore usually needs to be known to derive molar masses by gel permeation chromatography/size-exclusion chromatography-light scattering (GPC/SEC-LS). How to overcome the issue of unknown refractive index increments is outlined in this instalment of Tips & Tricks. In particular, a new approach to derive molar masses by GPC/SEC-LS that requires only the molar mass of a reference material, and not the dn/dc of the sample or of the reference material, is introduced.
Gel permeation chromatography/sizeâexclusion chromatography (GPC/SEC) is a powerful separation technique that helps to determine molar mass distributions and molar masses of polymers and macromolecules. However, GPC/SEC is a relative method requiring standards for calibration, which must be of identical chemical structure and topology as the samples to be analyzed. For samples where appropriate standards are not available, GPC/SEC with light scattering detection (LS) has become widely used. However, although light scattering is accepted to be an absolute method to determine molar masses, a variety of parameters must be known and instrument calibration is required. The last instalment of “Tips and Tricks” focused on the determination of the inter-detector volume, one of the parameters required to evaluate GPC/SEC-LS data (1).
Another crucial parameter required to determine molar masses by light scattering is the specific refractive index increment,
dn/dc, of the sample. This parameter is often unknown for the samples under investigation. This instalment of “Tips and Tricks” will therefore discuss possible strategies to handle this problem.
Basic Light Scattering
If a sample of sufficient low concentration is irradiated by a laser light beam, it usually scatters light. The Rayleigh ratio, R(0), that is, the scattering intensity in the direction of the incident beam resulting from a polymer of molar mass, M, at a concentration, c, which is dissolved in a solvent of refractive index, n0, can be written:
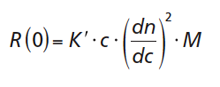
where [1]
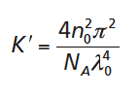
Here, λ0 and NA are the laser wavelength and Avogadro’s number, respectively. The parameter dn/dc is the specific refractive index increment of the polymer in the given solvent. The refractive index increment describes the difference in the refractive indices of the polymer and the solvent. If a sample disperse in molar mass is to be analyzed, M in equation 1 needs to be replaced by the weight average molar mass, Mw.
Strictly speaking, equation 1 is only valid at vanishing concentration and at zero scattering angle. The first requirement is usually met for the concentrations applied in typical GPC/SEC-LS.
For molecules larger than approximately λ0/20, the scattering intensity depends on the angle of observation, and the scattering intensities obtained at different scattering angles need to be extrapolated to zero scattering angle to derive the molar mass. From the angular variation of the scattered light the mean squared radius can be derived. The mean squared radius characterizes the polymer size in solution. For molecules smaller than approximately λ0/20, the angular dependence can be ignored and equation 1 is valid, irrespective of the angle of observation. Thus, the extrapolation to zero scattering angle is not required. For such small molecules, a light scattering detector with a single angle is sufficient to derive molar masses (2).
In the following we will assume that all intensities and the corresponding signal areas corresponding to the light scattering instrument are extrapolated to zero scattering angle.
For GPC/SEC-LS experiments, equation 1 and the above discussion holds true for each elution volume.
Offline Determination of dn/dc
From equation 1 it becomes clear that knowledge of the specific refractive index increment is essential to derive molar masses from light scattering. By measuring the refractive index of the solvent and for a series of different sample concentrations, the refractive index increment dn/dc can be derived by plotting the refractive index difference between sample solution and pure solvent versus sample concentration. The slope of the plot yields dn/dc. An example is shown in Figure 1.
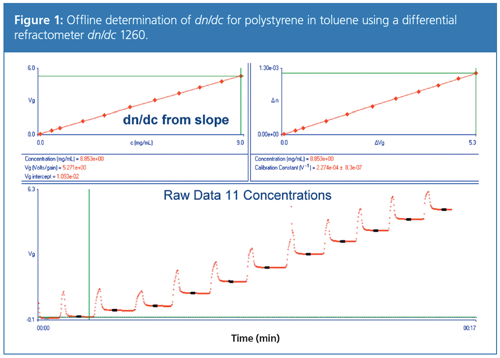
However, the precision of a simple Abbè refractometer is insufficient to derive dn/dc with the required accuracy. A differential refractometer is therefore required. Another problem of dn/dc determination arises from the fact that in mixed solvents or salt solutions, the macroscopically determined dn/dc is not identical to the refraction index difference responsible for light scattering (3–6). Therefore, dialysis of the sample solutions is required before dn/dc determination in ternary systems. This makes correct dn/dc determination in ternary systems labourâ and time-intense, raising the costs for each analysis.
Online dn/dc Determination Using a Calibrated RI Detector
An alternative is the use of a calibrated refractive index (RI) detector in a GPC/SEC-LS experiment. As in most GPC/SECâLS experiments, an RI detector is used in conjunction the LS instrument to determine the concentration at each GPC slice; no additional investment is needed. However, the refractometer needs to be calibrated in the solvent the GPC/SEC experiment is performed in. This calibration is done by injecting a known amount of a reference sample, for which dn/dc is known in the eluent applied. Often the same sample is also used to calibrate the light scattering detector itself.
Since the response of the RI detector is proportional to the product of concentration and dn/dc, the total area under the RI signal (ARI) can be written:
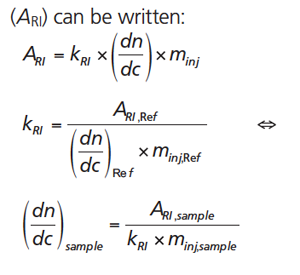
[2]
Here, minj is the total injected mass, which can be calculated from the injected concentration and the injection volume (minj = cinj × Vinj). kRI is the instrument constant of the RI detector.
According to the second line of equation 2 the instrument constant is derived by dividing the area of the RI signal by the product of injected mass and the reference material’s (Ref) dn/dc. Once the instrument constant is known, the injection of a known amount of the analyte (sample) allows determination of the analyte’s dn/dc using the last part of equation 2. The requirements that need to be fulfilled to use the approach of a calibrated RI detector are:
1. A reference material of known dn/dc in the solvent under investigation must be available.
2. Both analyte and reference material must elute completely from the column at the given chromatographic conditions.
However, in GPC/SEC the use of solvents containing additives or salts is not unusual. For these solvent systems-and sometimes even for pure solvents-a suitable reference material of known dn/dc is often not available. In this case it is difficult to apply this approach.
GPC/SEC-LS Without Knowing Any dn/dc
A new approach to calibrate GPC/SEC-LS setups has been reported recently (7). The approach allows the complete GPC/SEC-LS setup to be calibrated based solely on the molar mass of a reference material. Neither the dn/dc of the analyte nor of the reference material is required. Also, the calibration can be performed even if the refractive index of the solvent at the laser wavelength is unknown.
It was shown that the following relation holds true for GPC/SEC-LS setups with RI detection if the sample and the reference material are run under the same conditions (7).
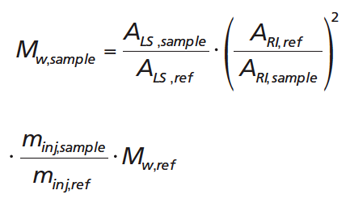
[3]
Here ALS,ref and ALS,sample are the areas of the light scattering peaks of the reference material and the sample, respectively. Likewise ARI,ref and ARI,sample correspond to the RI peak areas. The injected masses of reference material and sample are denoted minj,ref and minj,sample, and can be calculated from the injected concentration and injection volume. Mw,ref is the weight average molar mass of the reference material.
According to equation 3 only the molar mass of the reference material and the injected masses need to be known. The detector areas are measured.
Although equation 3 is written for the weight average molar mass of the sample, it can similarly be applied to calculate the molar mass at each elution volume. It should be stressed that the required reference material need not be of identical chemical structure to the sample being analyzed to derive the correct molar mass. This is in distinct contrast to a conventional GPC/SEC calibration, where the standards must be chemically identical to the analyte.
However, it should also be stressed that equation 3 was obtained assuming full recovery for the sample and for the reference material-similar to equation 2.
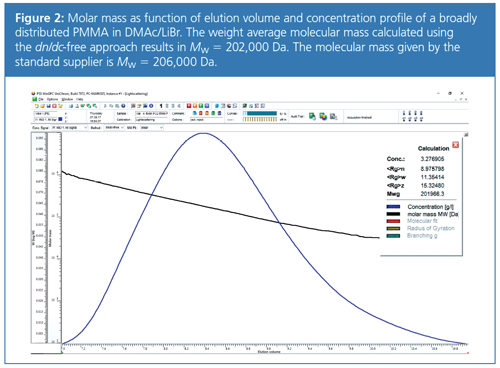
In Figure 2 the concentration profile and the dependence of molar mass on elution volume are shown for a broadly distributed PMMA in DMAc/LiBr. The molar masses were derived using the dn/dc-free approach. The evaluation of molar masses for the broadly distributed PMMA results in Mw = 202,000 Da, which is in good agreement with the expected molar mass of 206,000 Da. The evaluation of a large number of polymers of different chemical structure in various solvents revealed that the results of the dn/dc-free evaluation are of equal accuracy to those obtained using a calibrated RI detector (7).
Summary
- Molar mass determination by GPC/SECâLS requires the detector responses to be calibrated.
- dn/dc is a crucial parameter affecting the molar masses determined by GPC/SEC-LS.
- Offline dn/dc determination requires a separate instrument. When solvent mixtures or salt–additive containing solvents are applied, a correct dn/dc determination requires dialysis, which makes offline dn/dc determination labour intensive.
- Online dn/dc determination requires a calibrated RI detector and assumes complete recovery of the injected sample and the reference used to calibrate the setup. To calibrate the RI detector a material with known dn/dc in the eluent applied is required, making calibration difficult.
- A recently introduced method requires only the molar mass of the reference material and complete recovery of sample and reference material. Neither dn/dc of the sample nor of the reference material is needed, making the method easy to apply.
References
- D. Held and W. Radke, The Column13(11), 9–14 (2017).
- P. Kilz and D. Held, The Column5(4), 28–32 (2009).
- M.B. Huglin, Light Scattering from Polymer Solutions (Academic Press, London/New York, 1972).
- R. Brüssau, N. Götz, W. Mächtle, and J. Stölting, Tenside Surf. Det. 28, 396–406 (1991).
- D. Held, The Column5(2), 17–20 (2009)
- B. Maret, A. Crépet, C Faye, L. Garrelly, and C. Ladavière, Macromol. Chem. Phys.216, 95−105 (2015).
- S. Lavric, J. Preis, C. Rosenauer, and W. Radke, J. Chrom. A. In Press (2017). DOI: 10.1016/j.chroma.2017.09.011
Wolfgang Radke studied polymer chemistry in Mainz, Germany, and Amherst, Massachusetts, USA, and is head of the PSS application development department. He is also responsible for instrument evaluation and for customized trainings.
E-mail: WRadke@pss-polymer.comWebsite:www.pss-polymer.com

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.














