Determination of Polychlorinated Biphenyls in Water Samples Using a Needle Trap Device Combined with Gas Chromatography
In this study, a fiber-packed needle trap device (NTD) was developed by packing heat-resistant fibers with a polyethylene glycol sol-gel coating into a 21-gauge, stainless steel needle. The polyethylene glycol sol-gel coating has numerous advantages, including uniform roughness and a large specific surface area. The prepared NTD was used for headspace extraction of five polychlorinated biphenyls (PCBs) in water samples, determined by gas chromatography with a flame ionization detector (GC-FID). The main experimental parameters, including the extraction and desorption conditions, ionic strength, and fiber bundles, were investigated to improve the extraction efficiency. After optimization, satisfactory linearity (r > 0.99) in the concentration range of 0.02–500 μg/L was obtained, and the enrichment factor of NTD for the five PCBs was between 1150 and 9537 times. The limit of detection (S/N = 3) of five PCBs were measured in ranges of 0.0021–0.01 μg/L. Furthermore, the fiber-packed NTD has excellent durability, and can be reused for 60 cycles. After being stored at room temperature for three days, the storage ability of the NTD had a loss of PCBs less than 10%, and the relative standard deviation (RSD) was less than 10%. When analyzing the PCBs in real water samples, good accuracies (spiked recoveries were in the range of 92.19–98.56%) and precision (the RSD was lower than 12.8%) was obtained.
Polychlorinated biphenyls (PCBs) are compounds of the halogenated aromatic group. Owing to excellent chemical and thermal stability, PCBs are widely applied in diverse commercial production. However, the direct discharge of industrial waste and careless disposal accidents and chemical waste disposals results in the accumulation of PCBs in the environment. PCBs have become the main persistent organic pollutants found in diverse environmental matrices (1,2). Long-term exposure to PCBs can cause harmful effects on people, such as acne, rashes, and liver damage. A few studies have indicated that PCBs are associated with liver cancer and bile duct cancer (3,4). There are 15 PCBs that have been thought as probable carcinogens to humans by the U.S. Environmental Protection Agency (EPA) and the International Agency for Research on Cancer (IARC) (5). Therefore, it is necessary to monitor PCBs in the environment to assess their potential harm to people. However, the direct detection of PCBs in the environment is hard, owing to the complex matrix effect and their trace levels. Thus, effective sample pretreatment techniques should be developed.
Solid-phase microextraction (SPME) (6,7) as a simple sample preparation method has been successfully used for analyzing PCBs in aqueous samples (8,9). However, commercial SPME fibers have some drawbacks, such as fiber fragility, limited coating options, poor thermal stability, and limited sorption capacity (10,11). Needle trap devices (NTDs) and their ability to overcome the limitations of SPME were first mentioned in 2001 by Pawliszyn and colleagues (12). Various NTDs with different sorbents, such as metal-organic frameworks (MOFs), organic composites, nanoparticles, and carbon nanotubes, were developed for extracting aromatic amines (13), phenols (14), benzene, toluene, ethylbenzene, and xylene (BTEX) (15), and polycyclic aromatic hydrocarbons (PAHs) (16,17) from environmental samples. Fiber-packed needles, another type of NTD, have been prepared by packing polymer-coated fibers as the extraction medium into a stainless steel needle (18). Some fiber-packed needles with different polymer coatings have been prepared for the extraction and separation of some pollutants (19,20). Preparing an NTD with packed fibers not only makes the coating uniform, but it also improves the permeability of the device, and the fiber coating is protected by stainless steel so that it has a good service life, high thermal stability, and strong mechanical strength (21). Currently, however, fiber-packed NTDs are rarely used in the analysis of PCBs.
For fiber-packed NTDs, the good thermal and chemical stability of fiber coatings plays a vital role in target enrichment. Sol-gel coating technology presented by Malik and coworkers (21,22) has been used to prepare sorbents for the NTDs (23,24). In this method, the surface treatment, coating, and immobilization were effectively combined together (25). Because of the stable chemical binding of the coating to the substrate surface, sorbents coated using sol-gel technology have greater chemical and thermal stability (26–28). In addition, the porosity structure formed not only increases the surface area, but it also allows for adjustment of the coating thickness (29,30).
In the study presented here, a novel, fiber-packed NTD was prepared by the sol-gel method to extract PCBs from water samples. A three-dimensional (3D) polymer was prepared with polyethylene glycol (PEG) and methyltrimethoxysilane (MTMS) in an acid condition. Then, the fiber coating step was achieved by coating the polymer on the surface of high temperature-resistant Zylon fibers. The preparation of the coating was easy and inexpensive. The device was then applied to the analysis of PCBs combined with gas chromatography (GC). The effect of certain conditions (such as extraction time and temperature, desorption time and temperature, and ionic strength) on the extraction efficiency was studied. Moreover, the storage performance, service life, analytical performance (such as limit of detection [LOD] and limit of quantification [LOQ]), and recoveries of the proposed NTD were evaluated. Finally, five PCBs in water samples were successfully detected by this method.
Experimental
Instrumentation and Materials
Methyltrimethoxysilane, polyethylene glycol (PEG), trifluoroacetic acid (TFA), acetone (CH3COCH3), and sodium chloride were purchased from Aladdin Reagent Co. Ltd. All solvents used here were analytical-reagent grades. Standards of polychlorinated biphenyls (PCBs), including 2,4,4’-trichlorobiphenyl (PCB28), 2,2’,5,5’-tetrachlorobiphenyl (PCB52), 2,3,4,4’,5-pentachlorobiphenyl (PCB118), 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB153), and 2,2’,3,4,4’,5,5’-heptachlorobiphenyl (PCB180), were bought from Dr. Ehrenstorfer in Germany. The standard solutions (1.0 g/L) of PCBs in acetone were stored in the refrigerator at a temperature of 4 °C. Ultrapure water was processed by a Milli-Q system (Millipore). The heat-resistant Zylon fibers were supplied from Toyobo. The 21-gauge stainless steel needle was 5 cm in length, and had a high-temperature resistance above 300 °C.
GC Analysis
An Agilent gas chromatograph (7890B) instrument was used for analyzing the components, which was equipped with a flame ionization detector (FID) system. An HP-5 capillary column (30 m × 0.32 mm i.d.) with a 0.25 μm film thickness used for the separation. As a carrier gas, ultrapure N2 had a flow rate of 6.5 mL/min. The splitless injection was adopted. The GC injector temperature was 290 °C, and the detector temperature was 300 °C, respectively. The initial oven temperature was set at 100 °C before being increased to 250 °C at a rate of 5 °C/min.
Preparation of Fiber-Packed Needle Trap Device
Pretreatment of Zylon Fibers
The Zylon fibers were processed according to our previous study (31). Briefly, a bundle of Zylon fibers was immersed in an acetone solution for 6 h. After washing the fibers with distilled water, the fibers were dried with nitrogen. Then, the fibers were processed in a hydrogen chloride solution (0.1 mol/L) for 1 h, and dried for reserve.
Preparation of Coating Solution
Polyethylene glycol (200 mg as coating polymer) was weighed and dissolved in 200 μL of acetone in a centrifuge tube. Subsequently, 400 μL of methyltrimethoxysilane and 150 μL of TFA (95%), which served as an acidic catalyst into the centrifuge tube, were added and mixed well. After being ultrasonically agitated for 5 min, the prepared solution was centrifuged for 10 min at 12,000 rpm. Then, the supernatant was obtained.
Preparation of Needle Trap Device
The pretreated Zylon fibers were immersed in the prepared coating solution and kept at 30 °C for 30 min in the oven, followed by an additional 15 min at 100 °C. This coating step was repeated three times, and the new coating solution was used every time. Then, the Zylon fibers with the polymer coating longitudinally penetrated the hollow stainless steel needle. The prepared NTDs were put in a desiccator for 24 h, then aged at 280 °C for 4 h.
Extraction and Desorption
The stock solution was diluted with a 10 mL ultrapure water to prepare polychlorinated biphenyl (200 μg/L, PCB28, PCB52, PCB118, PCB153, PCB180) sample solution in a vial. The vial was closed with a Teflon septum and a headspace aluminum cap, and it was placed into the water bath at 80 °C. After magnetic stirring for 15 min, the gas–liquid equilibrium between the two phases in the vial was achieved. Then, the NTD connected to the syringe was inserted into the vial. The sample in the headspace was extracted by pushing and pulling the syringe repeatedly for 50 min. After extraction, the NTD was connected to the other syringe with 1 mL nitrogen, and then inserted into the GC injection port at 290 °C. Nitrogen was used to assist in the thermal desorption, and the desorption time was 4 min. The schematic of the device is shown in Figure 1.
FIGURE 1: Schematic of the extraction method using a NTD.
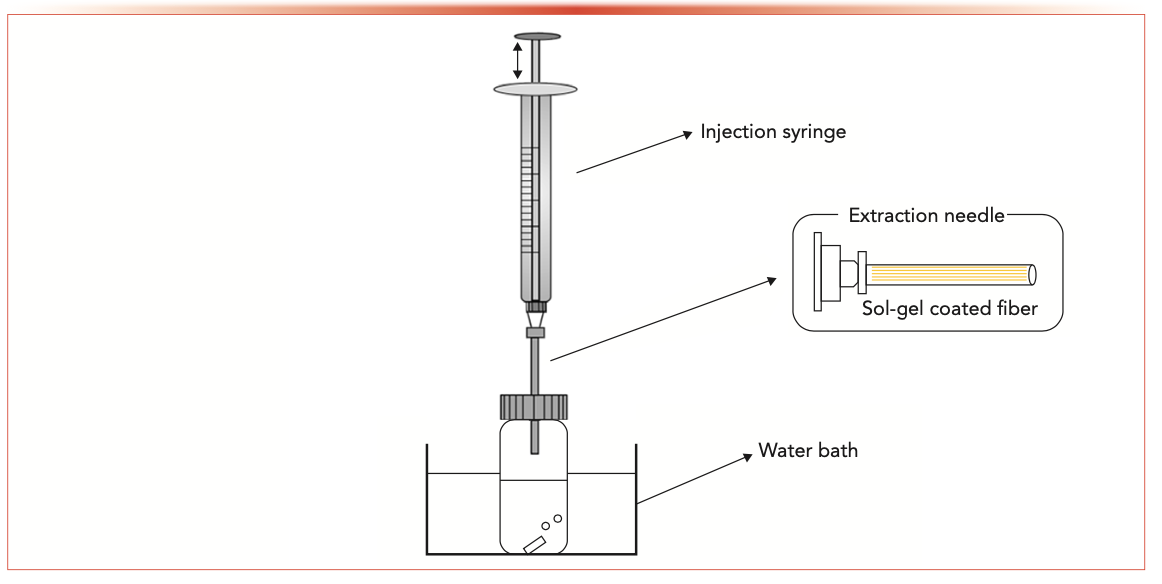
Results and Discussion
Characterization of the Fibers Coating
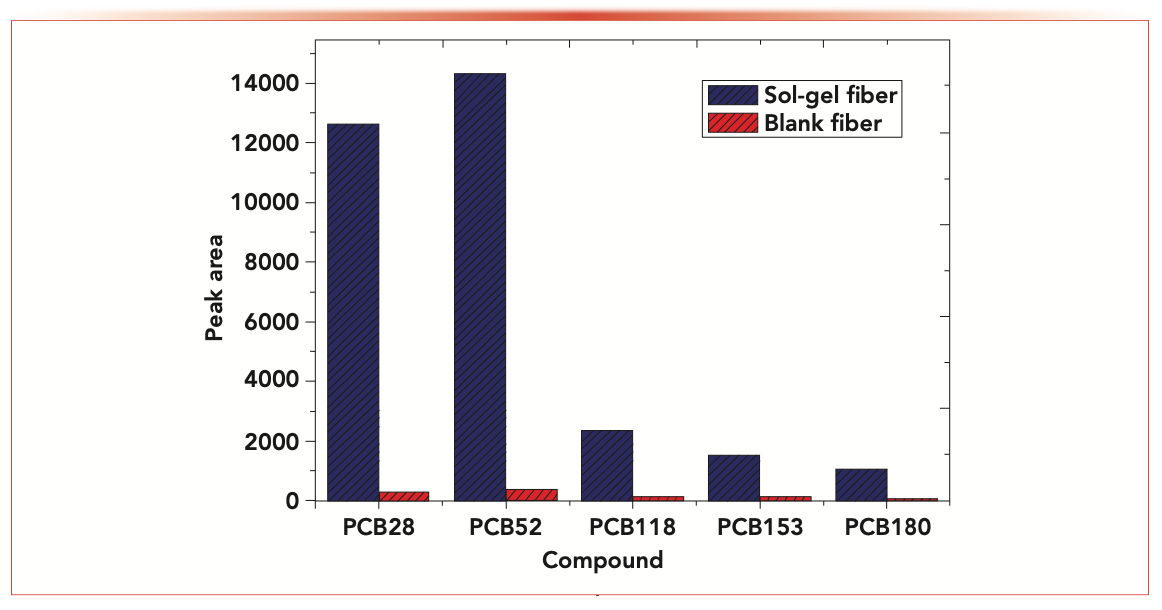
The surface morphological of the blank Zylon fibers and Zylon fibers coating were characterized by scanning electron microscopy (SEM) on a Zeiss Merlin Compact (Zeiss) instrument with the voltage at 25 kV. Figure 2a shows the surfaces of the blank Zylon fibers were smooth, but the coated fibers exhibited a rough surface which increased the surface area of the Zylon fibers (Figure 2b). As shown in Figure 3, the extraction efficiency between the coated fibers and blank fibers was in sharp contrast. The coated fibers had a high extraction capacity for PCBs. It can also be attributed to the chemical stability and the good thermal fiber coating obtained by the sol-gel technology (32,33).
FIGURE 2: SEM micrographs of (a) blank Zylon fiber and (b) Sol-gel PEG coating fiber (10,000-fold magnification).
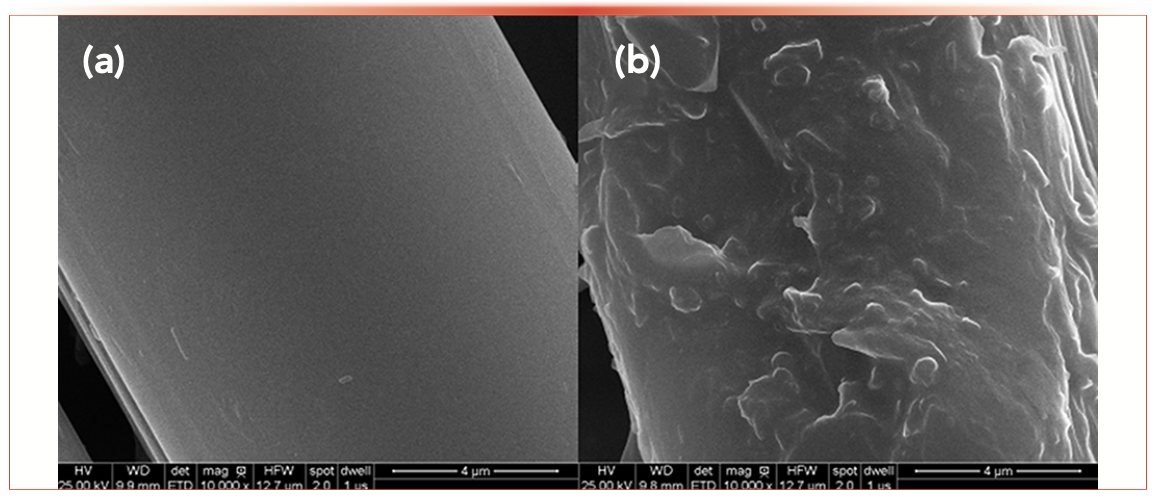
FIGURE 3: Comparison of extraction efficiency of blank Zylon fibers and Sol-gel PEG coating fibers for PCBs.
Optimization of the Conditions
Effect of Extraction Temperature
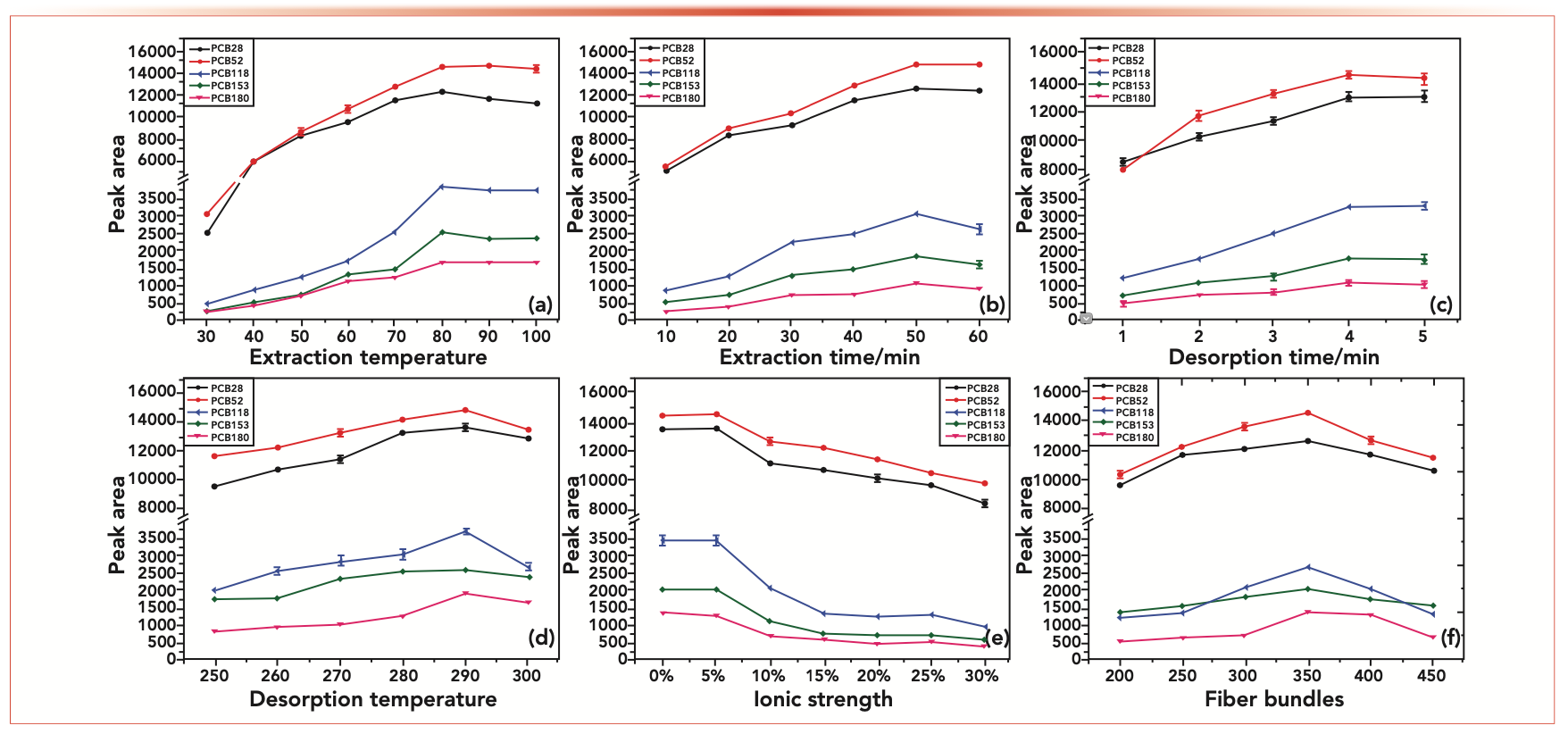
Extraction temperature is a key condition that influences the extraction efficiency of the NTD. The higher temperature is helpful in the diffusion of analytes in the headspace, which accelerated the transfer of the analytes into the fibers coating. However, the side effect of the extortionate temperature is that it changes the adsorption of the gaseous analytes on the NTD fibers, which makes the sensitivity lower. Therefore, the influence of extraction temperature was inspected from 30 °C to 100 °C. As displayed in Figure 4a, the highest peak areas of the five PCBs were found at 80 °C. Therefore, 80 °C was chosen as the best extraction temperature.
FIGURE 4: (a) Effect of extraction temperature, (b) extraction time, (c), desorption time, (d) desorption temperature, (e) ionic strength, and (f) fibers bundles on the extraction efficiency of PCBs.
Effect of Extraction Time
The headspace extraction is dependent on the equilibrium between the adsorbent coating and the sample. As a result, we studied the effect of the extraction time on the extraction efficiency of the NTD for the PCBs. The extraction was established within the range of 10–60 min at 80 °C with constant stirring. The results in Figure 4b showed that the extraction efficiency obviously increased from 10 min to 50 min, then decreased a little with an increase of extraction time. Therefore, the optimal extraction time was set at 50 min in the subsequent study.
Optimization of Desorption
Time and Temperature
The influence of desorption time on the extraction effect was researched from 1 min to 5 min. Figure 4c shows a longer desorption time can assist analytes delivery completely to the separation column. Therefore, 4 min was taken as the optimal desorption time for further study. The thermal desorption process was studied in a desorption temperature range of 250–300 °C. Figure 4d shows that with the temperature increase, the analyte peak area becomes larger. The maximum peak area was obtained at 290 °C. Then, the response of the peak area decreased significantly. So, 290 °C was chosen as the best desorption temperature.
Effect of Ionic Strength
The amount of salt added to the sample solution has varying effects on the equilibrium process. In this study, the effect of ionic strength on the extraction efficiency of the PCBs was researched in the sodium chloride concentration range of 0–30% w/v (Figure 4e). With the increase of the sodium chloride concentration, the peak area of PCB28 and PCB52 first increased slightly and then decreased significantly, whereas the peak area of the other three samples decreased continuously with the sodium chloride concentration increase. It could be because of salting out and competitive effects. Salt inhibits the solubility and distribution coefficient of the tested substance in aqueous solutions.
However, high salt concentration results in competitive adsorption with the analytes, which may make the adsorption performance of the adsorbent significantly reduced (3,11). Therefore, in the following study, no sodium chloride was added to the sample solution.
Effect of Fibers Bundles
The number of fibers bundles was also a factor affecting the extraction efficiency. Therefore, the influence of the number of fibers bundles on extraction efficiency in a range from 200 to 450 bundles was studied (Figure 4f). The results showed that the extraction efficiency increased with the amount of fibers bundles in the range of 200–350 bundles. Although the number of fibers bundles exceeded 350, the extraction efficiency is reduced because the excessive fibers bundles will affect the permeability of the device. Therefore, the number of fiber bundles was 350.
Method Validation
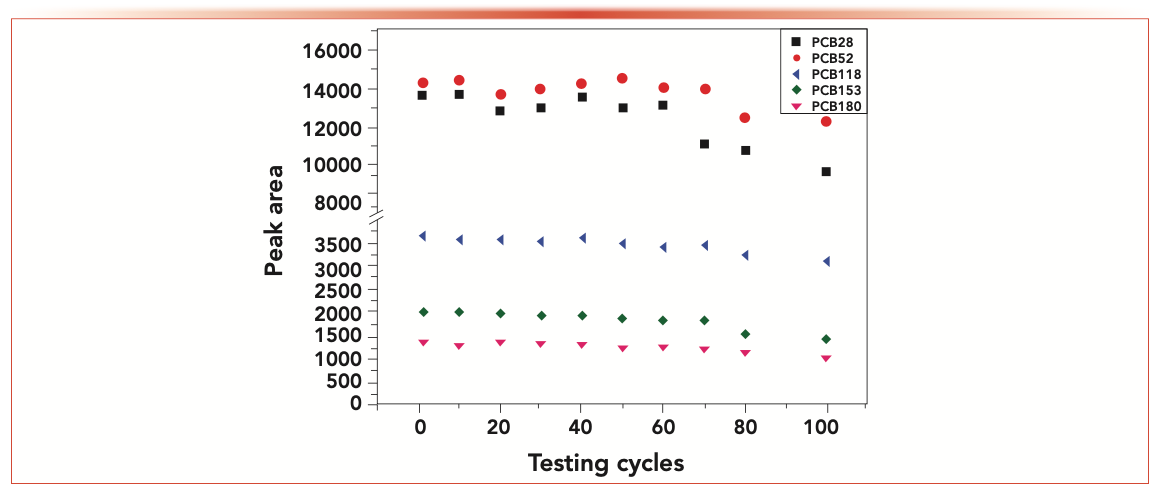
The applicability of the explored NTD for extracting five PCBs from water samples was evaluated. The analytical characteristics include the linearity, LOD, and repeatability was studied to validate the proposed method under optimal conditions. Table I lists the results. The linearity range of PCB28, PCB52, and PCB118 was 0.02–500 μg/L, and PCB153 and PCB180 was 0.05–500 μg/L with the correlation coefficient (r) between 0.9937 and 0.9999. The enrichment factors of NTD for the five PCBs were 8934, 9537, 2440, 2210, and 1150, respectively. The LOD (S/N = 3) was in the range of 0.0021–0.01 μg/L and the LOQ (S/N = 10) was in the range of 0.0069–0.033 μg/L, respectively. The relative standard deviation (RSD) (n = 3) of repeatability was less than 5.37% for the one needle. Meanwhile, the needle-to-needle RSD ranged from 4.66–9.26%. Because the number and uniformity of fibers coating were slightly different, the RSD of needle-to-needle reproducibility was slightly larger than run-to-run repeatability, which was satis- factory in both cases. The same needle device was repeated 100 times for PCBs analysis (Figure 5). The extraction efficiency remained almost unchanged within 60 cycles.
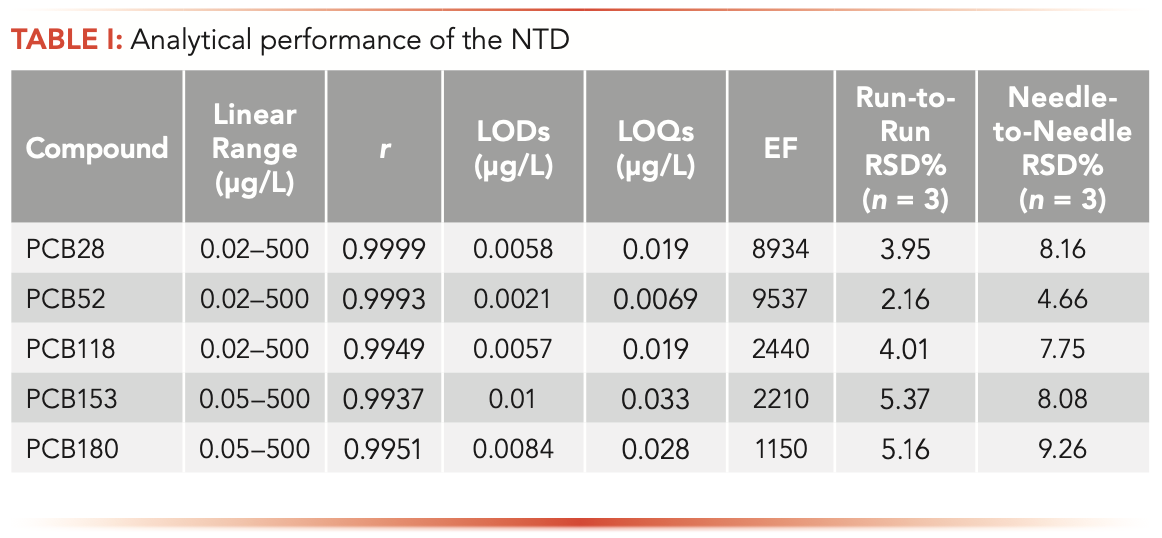
FIGURE 5: Service life of the needle trap device.
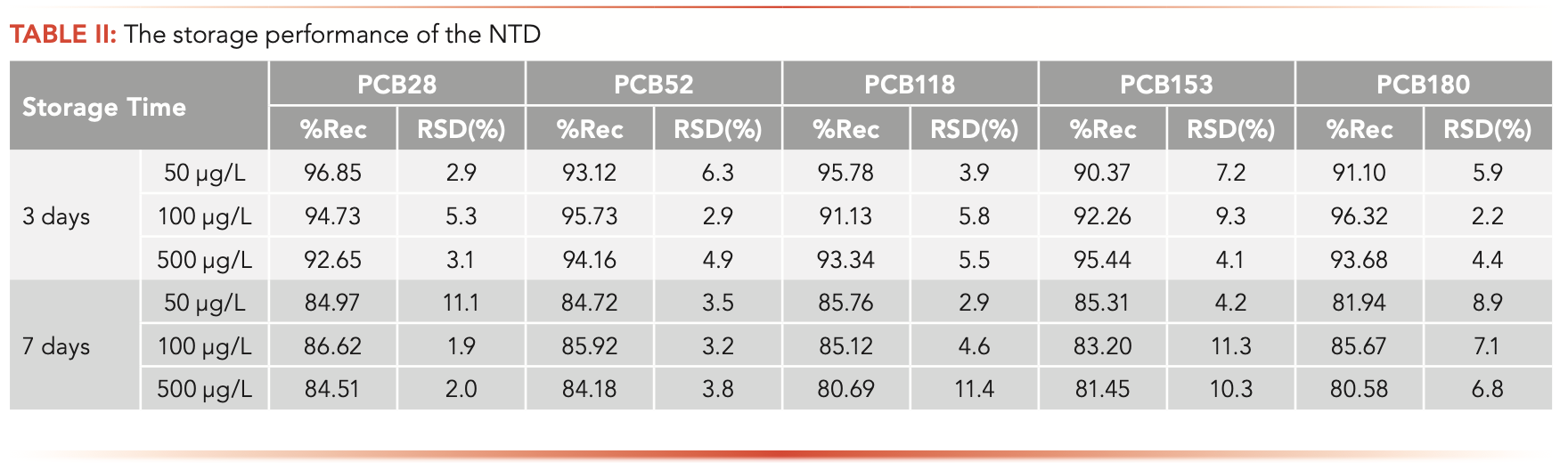
Storage Performance
To assess the storage performance of the device, the NTDs were used to extract the same amount of PCBs. Then, some of the needles with both ends sealed with PTFE caps were kept in laboratory conditions (room temperature 23 °C) for 3 d and 7 d. Recovery (%) of PCBs was obtained from the peak area before and after storage. As shown in Table II, the loss of PCBs in the device stored for 3 d had no obvious change, with recoveries between 90.37–96.85%. The corresponding extraction efficiency stored for 7 d decreased to some extent. However, the result was within the acceptable level.
The Actual Water Sample Analysis
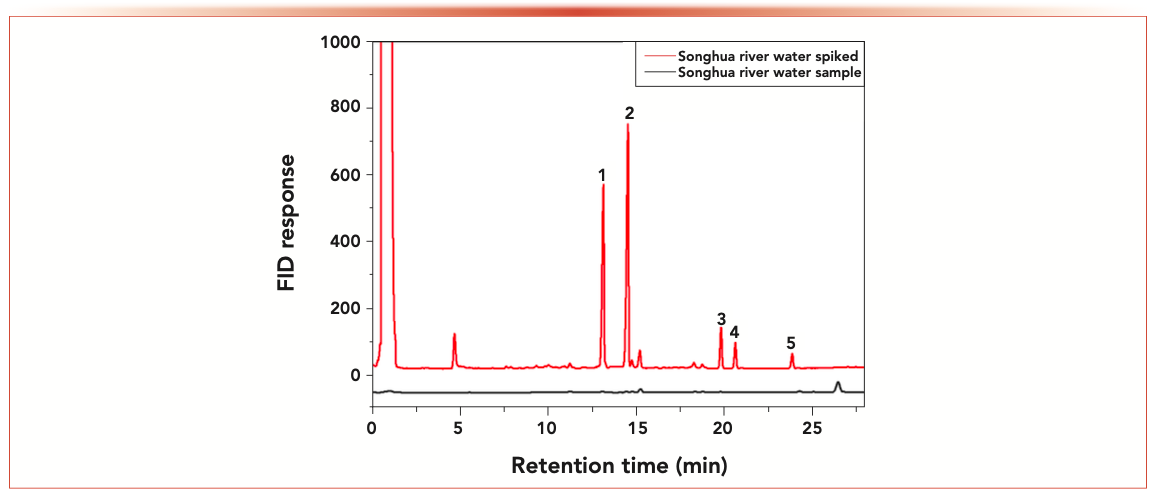
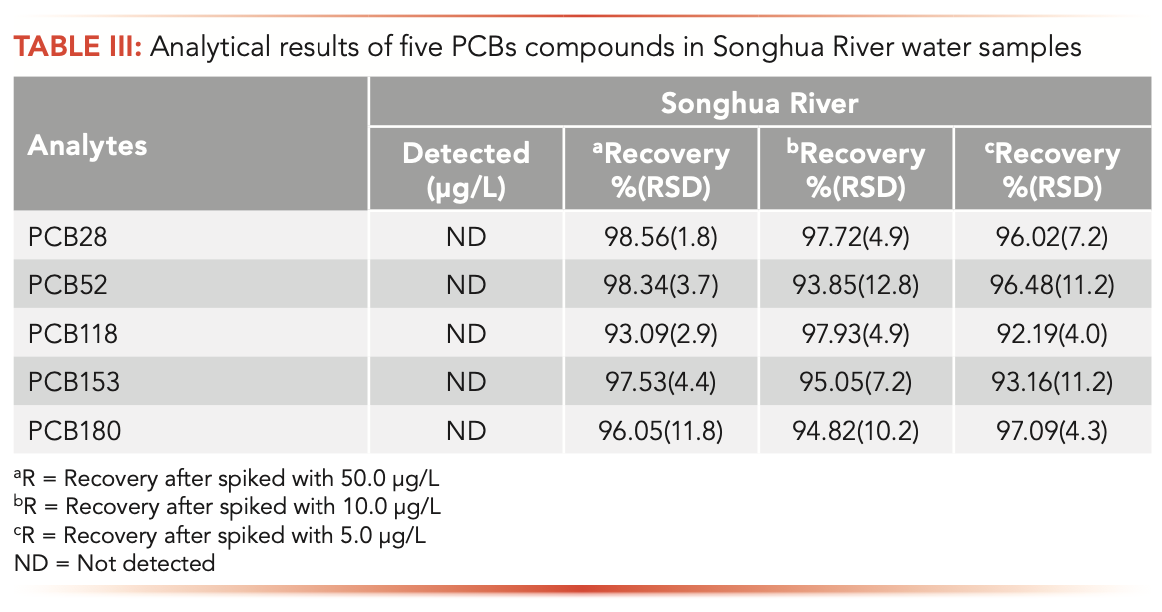
In this work, PCBs in the Songhua River water were extracted and analyzed by the NTD combined with a GC-FID detector. The gas chromatogram of PCBs in Songhua River water is shown in Figure 6. There were no PCBs found in Songhua River water. For accuracy measurement, 5, 10, and 50 μg/L of PCBs were spiked to Songhua River water. At each concentration, three replicated samples were performed. The results (Table III) showed that the recoveries of five PCBs Songhua River water were in the range of 92.19–98.56%, with RSDs 1.8–12.8%.
FIGURE 6: Chromatograms of the Songhua River water sample and the Songhua River water spiked with 50.0 μg/L PCBs. (1) PCB28; (2) PCB52; (3) PCB118; (4) PCB153; (5) PCB180.
Comparison with Other Methods
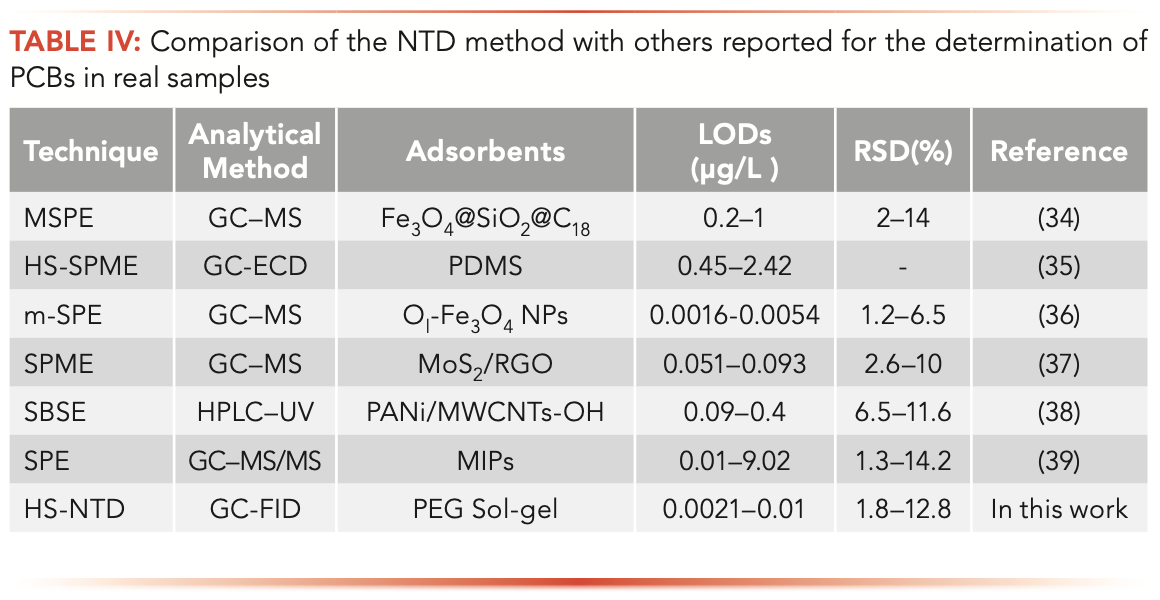
To demonstrate feasibility, the proposed performance was compared with other published research methods, and the results are expressed in Table IV. The comparable LODs and RSDs of the recoveries of the real sample was obtained in the present work. The device is appropriate for analyzing PCBs in water samples.
Conclusion
A novel NTD with sol-gel PEG-coated fibers in conjunction with GC was prepared and used to analyze PCBs in Songhua River water samples. The proposed method here can achieve a low LOD ranging from 0.0021 to 0.01 μg/L with good repeatability (RSD <9.5%). The thickness of the sol-gel coating could be adjusted by varying the coating time. The NTD provided good extraction efficiency and sample storage capacity for PCBs analysis. The method combined the NTD with GC and showed that it is a simple, inexpensive, and accurate tool for the trace PCB analysis in water samples.
Declaration of Competing Interests
There are no conflicts of interest to declare.
Funding
This work was supported by the Project of Science and Technology Development of Jilin Province (Grant numbers 20190303116SF, 202002008JC), the Project of Industrial Technology Research and Development of Jilin Province (Grant number 2020C028-1), and the Jilin Province Innovation and Entrepreneurship Funding Talent Project (Grant number 2020030).
Acknowledgments
We also want to acknowledge the Key Laboratory of Fine Chemicals of Jilin Province for instrument support in sample preparation and analysis.
References
(1) S.H. Safe, Crit. Rev. Toxicol. 24(2), 87–149 (1994).
(2) Z. Deng, X. Han, C. Chen, H. Wang, B. Ma, D. Zhang, et al, Mar. Pollut. Bull. 154, 111043 (2020).
(3) A. Derouiche, M.R. Driss, J.-P. Morizur, and M.-H. Taphanel, J. Chromatogr. A 1138(1), 231–243 (2007).
(4) S. Safe and O. Hutzinger, CRC Crit. Rev. Toxicol. 13(4), 319–395 (1984).
(5) Y. Wang, Y. Li, J. Zhang, S. Xu, S. Yang, and C. Sun, Anal. Chim. Acta. 646(1), 78–84 (2009).
(6) C.L. Arthur and J. Pawliszyn, Anal. Chem. 62(19), 2145–2148 (1990).
(7) J. Zheng, K. Wang, E. Luo, D. Wu, F. Zhu, R. Jiang, et al, Anal. Chim. Acta. 884, 44–51 (2015).
(8) M. Llompart, K. Li, and M. Fingas, Anal. Chem. 70(13), 2510–2515 (1998).
(9) J.-X. Guo, H.-L. Qian, X. Zhao, C. Yang, and X.-P. Yan, J. Mater. Chem. A 7(21), 13249–13255 (2019).
(10) M.R. Azari, A. Barkhordari, R. Zendehdel, and M. Heidari, Microchem. J. 134, 270–276 (2017).
(11) Y. Guo, X. He, C. Huang, H. Chen, Q. Lu, and L. Zhang, Anal. Chim. Acta 1095, 99–108 (2020).
(12) J.A. Koziel, M. Odziemkowski, and J. Pawliszyn, Anal. Chem. 73(1), 47–54 (2001).
(13) A.A. Alinaghi Langari, A. Firoozichahak, S. Alizadeh, D. Nematollahi, and M. Farhadian, RSC Advances 10(23), 13562–13572 (2020).
(14) A. Firoozichahak, A. Bahrami, F.G. Shahna, S. Alizadeh, D. Nematollahi, and M. Farhadian, J. Sep. Sci. 43(5), 1011–1018 (2020).
(15) N. Saedi, A. Bahrami, F.G. Shahna, M. Habibi Mohraz, M. Farhadian, and S. Alizadeh, Biomed. Chromatogr. 34(4), e4800 (2020).
(16) S. Soury, A. Bahrami, S. Alizadeh, F.G. Shahna, and D. Nematollahi, Microchem. J. 148, 346–354 (2019).
(17) Z. Ghalichi Zave, A. Bahrami, F.G. Shahna, and M. Farhadian, J. Chromatogr. A 1602, 74–82 (2019).
(18) K. Kędziora and W. Wasiak, J. Chromatogr. A 1505, 1–17 (2017).
(19) Y. Saito, I. Ueta, M. Ogawa, A. Abe, K. Yogo, S. Shirai, and K. Jinno, Anal. Bioanal. Chem. 393(3), 861–869 (2009).
(20) I. Ueta, Y. Saito, N.B.A. Ghani, M. Ogawa, K. Yogo, A. Abe, et al, J. Chromatogr. A 1216(14), 2848–2853 (2009).
(21) M. Ogawa, Y. Saito, S. Shirai, Y. Kiso, and K. Jinno, Chromatographia 69(7), 685–690 (2009).
(22) D. Wang, S.L. Chong, and A. Malik, Anal. Chem. 69(22), 4566–4576 (1997).
(23) M. Heidari, A. Bahrami, A.R. Ghiasvand, F.G. Shahna, and A.R. Soltanian, Anal. Chim. Acta 785, 67–74 (2013).
(24) M. Heidari, A. Bahrami, A.R. Ghiasvand, F.G. Shahna, and A.R. Soltanian, Talanta 101, 314–321 (2012).
(25) Z. Zeng, W. Qiu, and Z. Huang, Anal. Chem. 73(11), 2429–2436 (2001).
(26) Z. Wang, C. Xiao, C. Wu, and H. Han, J. Chromatogr. A 893(1), 157–168 (2000).
(27) L. Yun, Anal. Chim. Acta 486(1), 63–72 (2003).
(28) A. Spietelun, M. Pilarczyk, A. Kloskowski, and J. Namieśnik, Chem. Soc. Rev. 39(11), 4524–4537 (2010).
(29) X. Li, Z. Zeng, S. Gao, and H. Li, J. Chromatogr. A 1023(1), 15–25 (2004).
(30) J. Yu, C. Wu, and J. Xing, J. Chromatogr. A 1036(2), 101–111 (2004).
(31) X. Zhang, J. Chen, L. Lian, X. Wang, X. Guo, H. Chen, et al, Chromatographia 82(6), 953–960 (2019).
(32) M.S.W. Vong, N. Bazin, and P.A. Sermon, J. Sol-Gel Sci. Technol. 8(1), 499–505 (1997).
(33) Z. Zeng, W. Qiu, M. Yang, X. Wei, Z. Huang, and F. Li, J. Chromatogr. A 934(1), 51–57 (2001).
(34) M.-E.S. Synaridou, V.A. Sakkas, C.D. Stalikas, and T.A. Albanis, J. Chromatogr. A 1348, 71–79 (2014).
(35) C.H. Kowalski, G.A.D. Silva, R.J. Poppi, H. T. Godoy, and F. Augusto, Anal. Chim. Acta 585(1), 66–75 (2007).
(36) R.A. Pérez, B. Albero, J.L. Tadeo, and C. Sánchez-Brunete, Microchim. Acta 183(1), 157–165 (2016).
(37) F. Lv, N. Gan, Y. Cao, Y. Zhou, R. Zuo, and Y. Dong, J. Chromatogr. A 1525, 42–50 (2017).
(38) C. Hu, M. He, B. Chen, and B. Hu, J. Chromatogr. A 1394, 36–45 (2015).
(39) W. Li, Z.-M. Zhang, R.-R. Zhang, H.-F. Jiao, A.-L. Sun, X.-Z. Shi, and J. Chen, J. Hazard. Mater. 384, 121241 (2020).
Shuang Hou, Xiyue Wang, Lili Lian, Bo Zhu, Boying Yue, and Dawei Lou are with the Department of Analytical Chemistry at Jilin Institute of Chemical Technology, in Jilin, People’s Republic of China. Direct correspondence to: dwlou@hotmail.com or wangxiyue119@163.com

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













