Detection of Novel “Designer Drugs” Using Full-Scan GC–HRAM
Novel psychoactive substances pose a major challenge for forensic toxicology and drug-seizure laboratories because of the scale and speed at which these new “designer drugs” are entering the market. To keep pace with the threat, laboratories require robust full-scan detection techniques capable of both targeted drug screening and the identification of unexpected compounds too. Here, a full-scan gas chromatography (GC) coupled to high-resolution accurate mass (HRAM) approach for the profiling of known and unknown drugs of abuse is presented.
Photo Credit: Raimundo79/Shutterstock.com
Lisa Thomas, Thermo Fisher Scientific, Sunnyvale, California, USA
Novel psychoactive substances pose a major challenge for forensic toxicology and drug-seizure laboratories because of the scale and speed at which these new “designer drugs” are entering the market. To keep pace with the threat, laboratories require robust full-scan detection techniques capable of both targeted drug screening and the identification of unexpected compounds too. Here, a full-scan gas chromatography (GC) coupled to high-resolution accurate mass (HRAM) approach for the profiling of known and unknown drugs of abuse is presented.
To evade enforcement authorities and stay ahead of import and export laws, manufacturers of designer drugs are routinely modifying the chemical structure of the illicit compounds they produce. The detection of these novel psychoactive substances (NPS) presents a significant challenge for both forensic toxicology and controlled substance analysis laboratories, with 60% of U.S. forensic laboratories claiming NPS are a major contributor to drug chemistry caseload backlogs (1).
The recent and rapid emergence of the synthetic opioid fentanyl and its derivatives across North America highlights the scale of this challenge. A cheaper and more potent alternative to heroin, fentanyl has hit the U.S. particularly hard, with drug seizures increasing from 618 in 2012 to 4585 in 2014, according to the U.S. Centers for Disease Control and Prevention (CDC) (2). Unsurprisingly, the rapid increase in the volume of synthetic opioids entering the market has had a significant impact on drug abuse-related deaths, with the largest increase in opioid-related lethal overdoses a result of synthetic examples such as fentanyl.
Despite the best efforts of drug enforcement agencies, the global trade in synthetic opioids has evolved to stay one step ahead of the authorities. Suppliers are able to subtly modify the chemical structure of compounds such as fentanyl with relative ease, creating analogs such as acetyl fentanyl, which was considered legal to sell on the streets until it was also added to the scheduled list of illegal drugs in 2015 (3). Despite retaining a similar structure to their banned analogs, these new derivatives are easily able to evade detection by conventional targeted screening methods such as immunoassays or single or triple quadrupole mass spectrometry (MS). To keep pace with the threat of NPS, forensic chemistry and drug seizure laboratories require technology that not only detects known target compounds, but also identifies unexpected ones too.
The latest advances in Orbitrapâbased high-resolution accurate mass MS (HRAM) have proven to be valuable for full-scan screening of analytes in complex mixtures. This article demonstrates a nontargeted full-scan gas chromatography (GC) coupled to HRAM approach for the chemical profiling of drugs of abuse in urine. Using automated spectral deconvolution and spectral library matching software for forensic analysis, the identification of known and unknown drug compounds, including a fentanyl derivative not present in a spectral library at the time of testing, was achieved.
Experimental
Sample Preparation: Six urine samples (A–F) from real case investigations, provided by the Zurich Institute of Forensic Medicine at the University of Zurich, were analyzed. Sample extracts were prepared by adjusting 0.5 mL of urine to a pH of 5.2 by the addition of 100 μL of 0.5 M acetate buffer. After the addition of 50 μL glucuronidase–arylsulfatase, conjugate cleavage was performed at 60 °C for 3 h. A measure of 200 μL of ammonium buffer was added and the mixture liquid–liquid extracted with 1 mL of an ethylacetate:isopropanol:dichloromethane mixture (3:1:1 v/v/v). An 800-μL sample of the organic layer was evaporated to dryness, and the residue acetylated with 100 μL of an acetic anhydride–pyridine mixture (3:2 v/v) at 60 °C for 1 h. The acetylated mixture was evaporated and the residue then redissolved in 100 μL of ethyl acetate, which was used directly for GC–MS analysis.
Analytical Conditions: In all experiments, a Q Exactive GC Orbitrap GC–MS/MS system (Thermo Fisher Scientific) was used. Sample introduction was performed using a TriPlus RSH autosampler (Thermo Fisher Scientific), with chromatographic separation achieved using a TRACE 1310 GC system (Thermo Fisher Scientific) and a 30 m × 0.25 mm, 0.25-μm TraceGOLD TGâ1MS capillary column (Thermo Fisher Scientific).
GC Parameters: Injection volume: 1 µL; liner: split; inlet: 250 °C; carrier gas: He, 1.2 mL/min; split ratio: 20:1.
Oven Temperature Programme: Temperature 1: 50 °C; hold time: 1 min; temperature 2: 325 °C; rate: 10 °C/min; hold time: 8.5 min.
MS Parameters: Transfer line: 250 °C; ionization type: EI; ion source: 250 °C; electron energy: 70 eV; acquisition mode: full scan; mass range: 50–600 Da; resolving power: 60,000 FWHM at m/z 200; lockmass, column bleed: 207.03235 m/z.
Identification of Drugs of Abuse
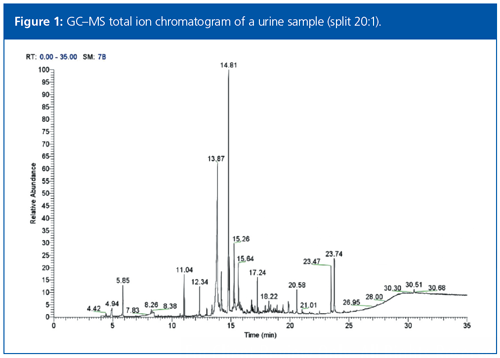
Full-scan data were acquired for the six acetylated urine samples. The total ion chromatogram (TIC) data for each sample was complex and possessed a high chemical background, as shown in Figure 1. This complexity was reduced using the automatic deconvolution functionality within the data acquisition and processing software. Deconvolution resulted in a cleaned spectrum for each peak, which contained only the ions that maximized at the same retention time.
Tentative compound identification was performed by searching the cleaned full-scan spectral data against the 2011 Maurer, Pfleger, and Weber mass spectral library of drugs, poisons, pesticides, pollutants, and their metabolites (4). Each data file was rapidly searched using a nominal mass library containing 8670 spectra of drugs, drug metabolites, contaminants, and biomolecules to generate a list of candidate compounds, with high-resolution data subsequently used to filter this list based on accurate mass. For each peak, the list of tentative hits was scored using a combination of a classical search index (SI) score and a high-resolution filtering (HRF) value corresponding to the percentage of the spectrum that could be explained by the chemical formula proposed from the best library match.
The total number of deconvoluted peaks extracted from the samples with a signalâto-noise (S/N) ratio greater than 100 ranged from 824 to 1574, with the number of peaks matching with at least one compound ranging from 169 to 263. These matched compounds not only comprised drugs of abuse, but also contaminants and biomolecules typical of complex urine samples. This list of matched compounds could be searched for drug compounds of most concern, with the results presented in Table 1.
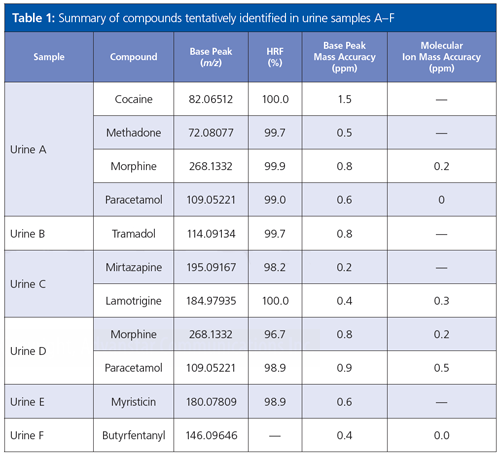
The use of both SI and HRF components for hit ranking significantly increased the level of confidence in analyte identification. For example, in the identification of the peak at 16.15 min in the TIC for sample E, the use of SI alone resulted in multiple acceptable matching compounds. When the HRF value was included and the list sorted based on the combined score, the drug molecule myristicin was found to be the top hit with a score of 74.3%, significantly higher than the next highest hits (< 51%). The identity of matched compounds could be further confirmed from the < 1 ppm mass accuracy of the ions.
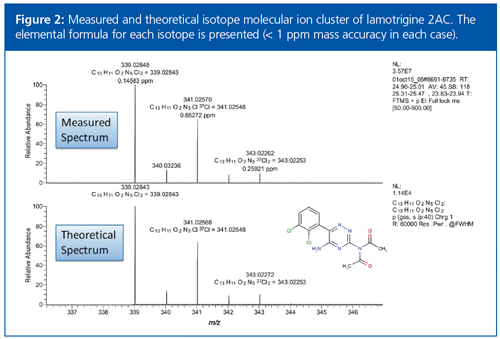
In addition to the accurate mass data for the molecular ion and fragment ions, the isotopic pattern of the molecular ion cluster could also be used to confirm analyte identities. For example, the anticonvulsant drug lamotrigine was identified in urine sample C by a positive match with lamotrigine 2AC (Figure 2). When the measured molecular ion cluster was compared with the theoretical pattern an almost identical match was found, with excellent mass accuracy for each isotope.
The accurate mass and isotope data are powerful criteria for compound identification, particularly because for many metabolites certified standards are not commercially available. This additional information reduces the evaluation time, producing results significantly faster than using nominal mass data alone.
The Importance of Dynamic Range
When performing chemical profiling of urine samples, compounds of interest can be present at both very high and ultratrace concentrations. For screening to be effective, detection methods must be able to provide the same level of performance regardless of analyte response.
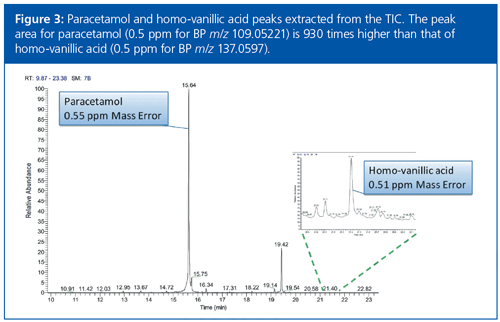
The chromatogram shown in Figure 3 for paracetamol and homo-vanillic acid highlights the potential of this GC–HRAM approach. The paracetamol peak area is approximately three orders of magnitude higher than that of homoâvanillic acid, yet the accurate mass performance remains at 0.5 ppm for both compounds.
Identification of Unknown Compounds
In sample F, a deconvoluted peak was identified that did not match with a compound in the spectral library. This peak had a base peak ion at m/z 146.09643, typical of fentanylâbased compounds. Alpha and beta methylfentanyl could be ruled out because there was no spectral match with these compounds in the library.
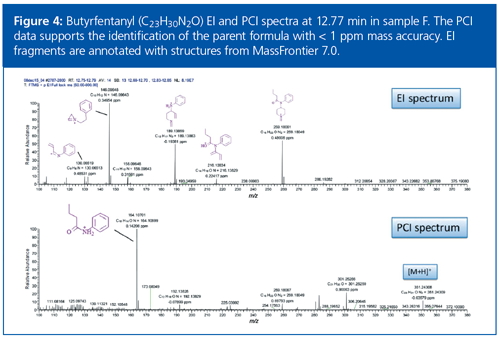
The accurate mass spectra obtained by electron ionization (EI) and chemical ionization (CI) were used to identify the [M+H]+ ion as m/z 351.24309, and a chemical formula of C23H31N2O was proposed with a mass difference of 0.03 ppm (Figure 4). This level of mass accuracy significantly reduced the number of formulae that needed to be investigated and increased confidence in the proposed assignment. Using this chemical formula to search the free onâline chemical database Chemspider, and suspecting that the compound was an analogue of fentanyl, the compound butyrfentanyl was proposed as an identity. This fentanyl analogue did not have an entry in the spectral library, and would explain the absence of a library match. Identification was further confirmed by software that theoretically fragmented butyrfentanyl and matched these fragments to the measured fragments with sub-1-ppm mass accuracy in the EI and CI spectra.
Conclusion
The continued rise in the number of firstâtime identified NPS poses a significant challenge for drug screening laboratories, who are not only faced with the task of detecting a broader range of known drugs of abuse, but also identifying unknown analytes of interest too. Using a GC–HRAM approach, several drugs of abuse in six complex urine samples taken from real case investigations were identified. Automated spectral deconvolution and library matching enabled rapid identification of known and unknown components with a high degree of confidence. This approach may be used to help drug screening laboratories reduce the burden of NPS identification on laboratory caseloads.
References
- National Forensic Laboratory Information System, 2013.
- Centers for Disease Control and Prevention, Increases in Fentanyl Drug Confiscations and Fentanyl-related Overdose Fatalities, 2015.
- Drug Enforcement Administration, Department of Justice, Schedules of Controlled Substances: Temporary Placement of Acetyl Fentanyl into Schedule I. Final Order, Federal Register, 2015.
- H.H. Maurer, K. Pfleger, and A.A. Weber, Mass Spectral Library of Drugs, Poisons, Pesticides, Pollutants and Their Metabolites (Wiley, 2011).
Lisa Thomas is senior director of marketing for the clinical and forensic markets in the chromatography and mass spectrometry division at Thermo Fisher Scientific.
E-mail:lisa.j.thomas@thermofisher.com
Website:thermofisher.com/forensics

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










