DESI, IMS, and Resurgent Challenges to HPLC–MS
LCGC North America
For great advancements in understanding made by intuitive leaps to be successful, they must withstand rational scrutiny.
For great advancements in understanding made by intuitive leaps to be successful, they must withstand rational scrutiny.
Liquid chromatography–mass spectrometry (LC–MS) has been increasingly indispensable in most analytical pursuits. Such acceptance would not have occurred without encouraging, early academic efforts, and also the efforts of early practitioners who pressed manufacturers to improve and extend electrospray's capabilities. I briefly described the commercialization of LC in its halcyon period of the early 1990s in a 1998 article (1).
When a sample is homogeneous, or the surface of the target analyte lends itself to interrogating, the ideal analysis would be simple and direct — no sample preparation. For LC–MS analysis, the sample must be amenable to the primary directive of LC: it must be soluble. Besides solubility issues, physicochemical properties, proton affinity, ion suppression, and other aspects of electrohydrodynamic reality demand temporal separation of one analyte from another. Successful ionization also depends upon the suitability of the droplet's surface. So practitioners have adapted by developing specialized skills and assembling appropriate reagents.

Michael P. Balogh
Electrospray Wings — for Molecular Elephants
The history of science reveals that great advancements in understanding are made by intuitive leaps at the frontiers of knowledge, not by intellectual walks along well traveled paths. But to be considered successful, those leaps must withstand rational scrutiny. Richard Cole (2) examines (and exposes) our uncertainties about the phenomenon of electrospray in his 2000 publication.
Those uncertainties over the exact mechanism of what we were seeing and using were significant. Malcolm Dole (3) first proposed the charge residue mechanism for electrospray in 1968. At about the same time, John B. Fenn was developing work that ultimately led, in 2004, to a Nobel Prize in Chemistry. (Incidentally, I apologize to Prof. Fenn for so impertinently appropriating the title of his acceptance lecture for this section.) Dole theorized that the repulsive forces in a charged droplet are offset by the droplet's surface tension. But as the droplet evaporates, its surface tension eventually can no longer oppose those repulsive forces. Thus, the droplet explodes, forming a multitude of smaller droplets. These coulombic fissions serially occur until droplets containing only a single analyte ion remain. When the solvent evaporates from the last droplet, a gas-phase ion is formed.
Iribarne and Thomson (4), however, proposed a different theory. In 1976, they introduced the ion evaporation mechanism, which also proposes that small droplets are formed by coulombic fission. In this theory, the electric field strength at the surface of the droplet is high enough to make it energetically favorable for ions to leave the droplet surface and go directly into the gas phase. As Cole argues, it is possible the two mechanisms actually can work in concert. The charge residue mechanism can dominate for masses higher than 3000 Da, while ion evaporation dominates for lower masses. In essence, a public debate over how electrospray ionization (ESI) ions come into existence has spanned more than 30 years, all the while that we have employed, widely and successfully, ESI.
Thus, despite the theoretical debate, the empirical evidence is compelling, and ESI is a proven and invaluable tool for MS analysis. Over the years, we have developed what we might call (borrowing an IT term) "workarounds" — the means to adapt to a technique's liabilities and shortcomings — to garner its benefits. In his Nobel Prize acceptance lecture for applying the principles of early ESI theory, Fenn (5) points out that Dole turned his interest elsewhere in the early 1970s, this after his "paper failed to persuade other investigators to confirm his experiments, which though simple in principle, were really very difficult and demanding in practice." Since then, however, the number of papers addressing the ESI mechanism has grown dramatically.
Many techniques we find in practice today had been known but not considered practical for many years, and they languished until new technologies unleashed their benefits. For instance, time-of-flight (TOF) technology dates back to the first half of the twentieth century (6–8). But it was not practical until data-handling electronics — high-speed digital signal processing — made it so. The same is true for LC–MS itself, because the early studies often despaired of what to do with the high liquid vapor volumes it produced. Just a few years ago, flow splitting to reduce the volume of liquid introduced into the mass spectrometer was common practice. Today, however, we can choose capillary-to-nanoscale delivery.
We often find that when we go about solving problems, our understanding and knowledge of a thing increases. Reducing LC liquid interference is such a case. Doing so resulted in the improved linearity of response in MS interfaces (7), and it happened, as it were, on the heels of efforts that proposed chemical expedients (8) or tried to explain the lack of linearity by theoretical means. Capillary liquid delivery, although not so robust until recently, clearly was advantageous, if not always easy. Perhaps the recent efforts coupling photoionization with nanospray flow rates similarly will sustain interest in atmospheric pressure photoionization as a potential "universal" ionization technology.
Ion mobility spectrometry (IMS) claims adherents because the technology brings a unique and desirable capability: simplicity. But the simplicity comes at the cost of limitation: as a gas-phase electrophoretic technique, IMS most often is relegated to gas-phase environments. It has not been employed widely in the condensed-phase world of LC. Nevertheless, its role in broader applications might prove better aligned with LC–MS analysis, as an adjunct component to the mass spectrometer.
Frequently, investigations of IMS's simplicity and direct usefulness are seen in cleaning validation (9). The U.S. Food and Drug Administration (FDA) requires manufacturers to verify cleaning to residue limits of 10 ppm or biological activity levels to 1/1000 of the normal therapeutic dose. In terms of surface area, this is equivalent to a range of 1–10 μg/cm2. IMS reportedly can achieve limits of detection of greater than 0.001 μg. It separates ions according to their mobility in a weak electric gradient field while opposing a flow of gas. Resolution is a product of the drift gas flow. Thus, ions traveling at different speeds (with typical drift times of 5–30 ms) can be separated according to size rather than mass-to-charge ratio differences.
The simplicity of IMS is evident in many handheld field applications in which ionization of airborne or gas-phase molecules occurs via radioactive beta emitters such as nickel-63 or americium-241. Large, laboratory IMS devices rely upon thermal desorption of the sample from a polytetrafluoroethylene substrate. The recorded acquisition is analogous to UV. That is, the resultant graph is proportionate to changes in the field and must be assessed relative to a known standard.
The disadvantage of IMS manifests itself in the need for further specificity of detection in other-than-targeted applications. In recent work that couples IMS and MS, ions are "gated" for introduction to the MS. But for LC–MS applications, issues can arise regarding the time needed for ions to make the transition from mobility device to mass spectrometer (10). Typically, the duration for acquiring a full scan covering a moderate mass range 50–300 m/z was 5 s. As the authors note, "for applications in which speed of analysis is critical, a time-of-flight mass spectrometer can be used instead." For those who are interested, the referenced paper provides a highly comprehensive review of IMS developments (11).
DESI and IMS
Novel analytical work constitutes a core element of the annual Conference on Small Molecule Science (CoSMoS), which highlights technology that has demonstrated sufficient potential to play a role in practice (12). Two researchers this year contributed their work in desorption electrospray ionization (DESI): Justin Wiseman of Purdue University, West Lafayette, Indiana, and Daniel Weston, a senior fellow at Nottingham Trent University, Nottingham, England. Weisman is one of the authors of a recent patent application on DESI. Weston, a contributing member of the National Initiative on Ion Mobility Spectroscopy (NIIMS) in the U.K., has been applying both DESI and IMS. Robert Cody, a scientist and co-inventor of a similar technology from JEOL-USA, Inc., Peabody, Massachusetts, contrasted and compared his contributed work on direct analysis in real time (DART).
DESI has been likened to matrix-assisted laser desorption ionization (MALDI) because it produces ions from surfaces through what is thought to be chemical sputtering (13). But unlike MALDI, DESI does not require an assist from a complex cocrystallization of a matrix with the analyte of interest. Various mechanisms have been postulated. One includes splashing charged nanodroplets from surface molecules, producing ESI-like spectra (direct evidence is given by a charge-state distribution similar to ESI). Another mechanism has gas-phase ions interacting with molecular species that typically are not ionized by ESI through electron, proton, and other ion exchanges.

Table I: Parameters are composites from presentations at CoSMoS by Wiseman and Weston. Note the angle of incidence to the sample/substrate and tip-to-inlet distance, which are often cited as critical. Note that some parameters (*) are expressed jointly as they are specified by the manufacturer.
DESI creates secondary ions directly from the surface of the material of interest. It typically is applied to an inert surface or the material itself. The Prosolia literature (Prosolia, Inc., Indianapolis, Indiana), derived from work in Graham Cooks' lab at Purdue University, mentions whole tomato skins, finished pharmaceutical product formulations, and human skin following ingestion of an over-the-counter product (14). Much of the early work (14) was developed on Thermo ion traps (Thermo Electron Corporation, Waltham, Massachusetts). But interest in the technology over just the past two years has prompted numerous others to investigate DESI — in essence for all types of mass spectrometers. Unlike ESI, which requires an analyte of interest to be in solution before converting it to gas-phase ions, DESI aims the equivalent of an ESI probe at the intended surface, at an angle (Figure 1). A mixture of liquid similar to that used in LC mobile phases (such as water–methanol) and controlled by operating parameters similar to those specified for ESI conditions, is then sprayed. Then, as in the case of ESI, the resultant ions enter the mass spectrometer's inlet. Table I shows parameters for DESI that are composites from presentations by Wiseman and Weston. Note the angle of incidence to the sample–substrate and tip-to-inlet distance, which often are cited as critical.
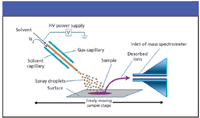
Figure 1: DESI schematic as described in the initial publication on the technology (15).
The differentiation that IMS drift times supply, in conjunction with DESI, provides a definitive candidate to the quadrupole TOF (Q-TOF) mass spectrometer for MS–MS without traditional LC separation or sample preparation. Despite this early stage of the technology's development, some limited semiquantitative claims can be made for sensitivity: 10–50 pg lysozyme [Cooks (12)] and a limit of detection (LOD) of 210 ng (700 pmol) in final dosage analysis using DESI–IMS–Q-TOF [Weston (12)]. Investigators from Cooks' group (15) have ascertained up to three orders magnitude linearity and precision to < 3% RSD.
Gary van Berkel, one of the early investigators of the DESI technology and a scientist at Oak Ridge National Laboratory, Oak Ridge, Tennessee, is also a recipient of the Biemann medal, awarded in 2004 by the American Society of Mass Spectrometry (ASMS). In a recent conversation, he suggested that the approach to DESI does not differ significantly from what we consider functional chemistry. His work so far has used thin-layer chromatography plates as the substrate and underscores the fact that we need to better understand surface interactions (15). Claims made of applicability beyond the more polar analytes common to ESI were supported in his investigations. Carotenoids, which typically are not amenable to ESI, were analyzed. According to van Berkel, it appears they might be ionized by electron-transfer processes during desorption or in the gas phase immediately following desorption. Recently published work by the Cooks group indicates additives in the mobile phase can play a role in enhancing response of specific target analytes (16).
To date, most of the work using DART has been performed on the JEOL Accutof, a TOF instrument (JEOL Ltd., Tokyo, Japan), but feasibility demonstrations, part of the commercialization effort by Ionsense, Inc. (Peabody, Massachusetts), includes other manufacturers. Like DESI, DART works at ambient pressure in open air. In the DART design (Figure 2), gas flows through the probe where an electrical discharge creates plasma. Lenses remove charged particles; a grid prevents ion–ion recombination at exit (17). The ionization and desorption mechanism is attributed to metastable (penning) ion production.
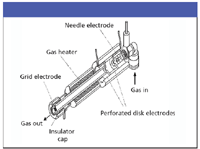
Figure 2: DART ionization device (courtesy Robert Cody, JEOL-USA, Inc.).
At this point in either process, the formed ions are undifferentiated by time. Daniel Weston, working in Colin Creaser's lab at Nottingham Trent University, U.K., has coupled IMS with DESI in his work using a Waters Q-TOF system modified with an IMS device (Figure 3). He has been able to selectively quantify analytes of pharmaceutical interest from tablet and cream-based samples with no sample preparation. Figures 4 and 5 are from one of his presentations (12), illustrating the combined advantage of IMS preselection of target ions for enhanced single MS applications as well as MS–MS. The pharmaceutical work has been accepted for publication, but is not in print as this column goes to press.
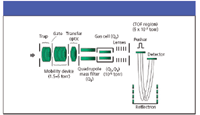
Figure 3: Schematic of IMS placement in Waters Q-TOF system (courtesy of Daniel Weston and Colin Creaser, Nottingham Trent University, U.K.).
This column is a good vehicle for passing on to a wide audience promising novel MS-related technologies that loom just over the horizon. But to speculate as to whether new technologies with more promise than practice will find their niche or fail would be risky. Nevertheless, optimism about the future of DESI applications does seem warranted. Similar to what we have seen in recent years with tissue-imaging applications of MALDI (18), DESI is apparently gentle enough for work directly from animal tissue, plant tissue, and biological materials. Direct testing of produce for pesticide residue, in vivo drug metabolites, forensics of trace materials, quality-control assays in manufacturing, and clinical assays including microorganisms and bacteria are all candidate applications.
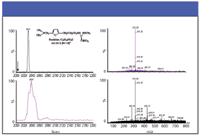
Figure 4: DESIâIMSâMS of Zantac 75 (GSK) showing the improvement (upper trace) of adding IMS as a further discriminator to single-quadrupole applications. Mobile phase: 49:49:2 (v/v/v) acetonitrileâwaterâformic acid; flow rate: 10 μL/min.
Clinical chemistry, in which folks are steeped in the culture of immunoassay procedures, is only now beginning to embrace the complexities of LC–MS (19). We have seen in European Union (EU) food safety practices that food residue analysis has crucial political and economic importance, and its scope and analytical sophistication are increasing (20). Performable by the rank-and-file practitioner, DESI can make residue analysis a direct, no-sample-prep technology with target-specific, disposable substrates.
A number of claims have been made about the depth and breadth of DESI. Through the continuing efforts of people such as those described in this column, we will see in the next couple of years how the technology fares. It is relatively simple and, as with ESI, works almost intuitively without requiring us to first understand the mechanics in detail to achieve well characterized, reproducible results.
Michael P. Balogh "MS — The Practical Art" Editor Michael P. Balogh is principal scientist, LC–MS technology development, at Waters Corp. (Milford, Massachusetts); an adjunct professor and visiting scientist at Roger Williams University (Bristol, Rhode Island); and a member of LCGC's editorial advisory board.
References
(1) M.P. Balogh, LCGC 16(2), 135–144 (1998).
(2) R. Cole, J. Mass Spectrom. 35, 763–772 (2000).
(3) M. Dole, L.L. Mack, R.L. Hines, R.C. Mobley, L.D. Ferguson, M.B. Alice, J. Chem. Phys. 49, 2240 (1976)
(4) J.V. Iribarne, B.A. Thomson, J. Chem. Phys. 64, 2287 (1976).
(5) J. B. Fenn, Electrospray Wings for Molecular Elephants, Les Prix Nobel, The Nobel Prizes 2002, ed. Tore Frangsmyr, Nobel Foundation, Stockholm (2003).
(6) W.E. Stephens, Phys. Rev. 69, 691 (1946).
(7) A. Cappiello, M.P. Balogh, G. Famiglini, F. Mangani, and P. Palma, Anal. Chem. 72(16), 3841–3846 (2000).
(8) F.R. Brown and W.M. Draper, Biol. Mass Spectrom. 20(9), 515–521 (1991).
(9) K. Payne, W. Fawber, J. Faria, J. Buaron, R. DeBono, and A. Mahmood, Spectroscopy 20(1), 24–27 (2005).
(10) B.H. Clowers and H.H. Hill Jr., Anal. Chem. 77, 5877–5885 (2005).
(11) A. Sysoev, A. Adamov, J. Viidanoja, R. Ketoia, R. Kostiainen, and T. Kotiaho, Rapid Commun. Mass Spectrom. 18, 3131–3139 (2004).
(12) Full text of 2005 CoSMoS presentations is available at www.CoSMoS2005.org/agenda.htm.
(13) Z. Takats, J.M. Wiseman, B. Gologan, and R.G. Cooks, Science 306, 371–473 (2004).
(14) www.prosolia.com.
(15) G.J. van Berkel, M.J. Ford, and M.A. Deibel, Anal. Chem. 77(5), 1207–1215 (2005).
(16) I.C. Rodriguez, Z. Takats, N. Talaty, H. Chen, and R.G. Cooks, Anal. Chem. (2005).
(17) R.B. Cody, J.A. Laramee, and H.D. Durst, Anal. Chem. 77, 2297–2302.
(18) M. Stoeckli, P. Chaurand, D.E. Hallahan, and R.M. Caprioli, Nature Med. 7, 493–496 (2001).
(19) M.P. Balogh, LCGC 23(8), 744–750 (2005).
(20) M.P. Balogh, LCGC 23(10), 1092–1099 (2005).


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












