Data Integrity in the GxP Chromatography Laboratory, Part III: Integration and Interpretation of Data
LCGC North America
Integration is the heart of the chromatographic process and is subject to regulatory scrutiny. What should be done to control integration and interpretation of the chromatographic runs?
This is the third of six articles on data integrity in a regulated chromatography laboratory. The first article discussed sampling and sample preparation (1), and the second looked at preparing the instrument for analysis and acquiring data (2). Now we focus our attention on the integration of the chromatographic data files that we acquired in the last article. Figure 1 shows what will be covered in this article.
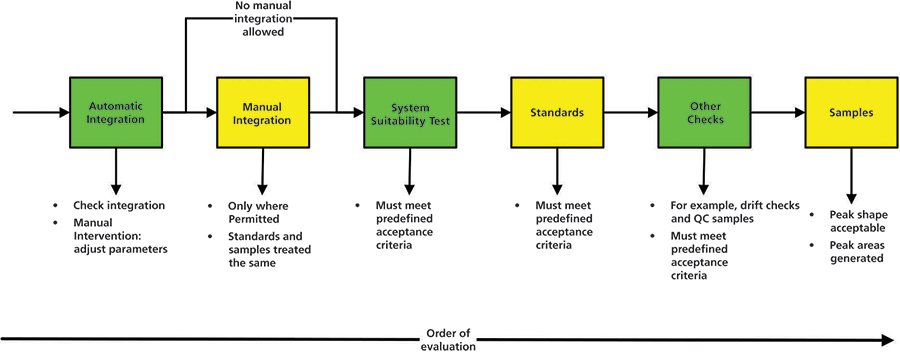
Figure 1: Scope and order of chromatographic integration.
Controlling Integration
Control of chromatographic integration is a key regulatory requirement as discussed in a recent book on the validation of chromatography data systems (3). Lack of integration control has resulted in several U.S. Food and Drug Administration (FDA) warning letters and 483 observations, such as one describing what FDA investigators found at Leiner Health Products (4):
“In addition, our investigators documented many instances with extensive manipulation of data with no explanation regarding why the manipulation was conducted. This manipulation would include changing integration parameters or relabelling peaks such that previously resolved peaks would not be integrated and included in the calculation for impurities . . .
“There was no Standard Operation Procedures (SOP) to describe the policy, standard practice, and circumstances under which manual integration would be allowed.
“There was no documentation of a justification for the manual integration.”
This is not an isolated case. Divi’s Laboratories received a warning letter in 2017 (5), stating “Your firm reintegrated multiple chromatograms to determine [redacted] levels; however, the parameters for the reintegration were not retained.”
The problem is that regulatory agencies want to see control of integration, specifically manual integration. However, we also need to acknowledge that chromatographic systems are dynamic by nature, and separations can change during a run. Thus, getting the right overall integration constantly throughout a run can be a balancing act. This situation is especially true with long overall runs comprising many samples and injections, with complex separations or biomolecules, where the peak shape is broad.
General Principles for Controlling Integration
Before going into detail for an SOP for chromatographic integration, we need to understand the general principles for good integration:
- Know how key parameters such as peak width and threshold impact the integration of a chromatogram (3,6).
- Never use a default method. Always develop the integration specifically for an analytical procedure.
- Have a robust and reliable analytical procedure; the sole purpose of a chromatography data system (CDS) is not to compensate for poor chromatography-good integration requires good chromatography.
- All integration, in the first instance, must be performed automatically.
- Chromatography is a comparative technique; therefore, standards and samples must be treated the same throughout the run.
- Given the regulatory concerns, only perform manual integrations under conditions permitted in your firm’s chromatography SOP or analytical procedures.
An SOP for integration will cover all the above points. However, there is no definition for manual integration. We propose the following definition: manual repositioning of the baselines, as opposed to manual intervention, which refers to changing integration parameters (3).
SOP for Integration of Chromatograms
Having established the fact that a chromatography procedure is a regulatory expectation (7), let us turn our attention to the content of such a procedure. We provide a master list of subjects and behavioral expectations. Depending on your organizational approach, some topics might be moved into the training material rather than the procedure. Nevertheless, the topic should be addressed to ensure consistency is practiced and to avoid the appearance of testing into compliance. We will use the previously discussed terms manual intervention and manual integration to describe nonautomated adjustments made to chromatograms by personnel (3,8).
Robust Chromatographic Integration SOP
The contents of an SOP on integration should include the following items:
1. Definitions: automatic integration, manual intervention, manual integration, analysis, method, test, sample, inhibit, peak masking, processing method. These are among many terms that must be defined for clarity in interpretation among personnel. This need becomes more critical as the number of users and laboratory sites is increased.
2. To set the foundation of the procedure, a set of fundamental behaviors and expectations must be introduced. Without these, the balance of the procedure is not achievable. These expectations must become part of training and include:
- Autointegration of all injection peaks is the expectation. Automatic integration reduces the data integrity risk to the organization, increases consistency in results, and significantly reduces the time to review chromatograms; therefore, manual interventions or integrations are accepted only after no algorithm can be developed to process chromatograms from this material.
- Disabling any audit trail or adjusting a computer system clock connected with the chromatography system is unacceptable.
- Performing any sequence of steps to avoid leaving an audit trail entry is unacceptable.
- Audit trail reason codes entered by users must provide sufficient detail to permit an inspector to reconstruct the sequence and actions performed by laboratory personnel.
- Postinjection data processing will follow a defined sequence: system suitability and criteria acceptance; reference standards and criteria acceptance; drift checks or other checks and criteria acceptance; and samples and other solutions. A prescribed order reduces the temptation to reject a chromatograph run because of undesirable sample results.
3. A file and data naming convention should describe the proper label for every type of material that will be injected into a chromatography system: suitability, standards, blanks, samples, control samples, drift checks, and so forth. There should be no place for analysts to use informal terms like test, wash, injection, or other such terms found in laboratories by inspectors (9,10). In a regulated environment every injection has a defined purpose; therefore, every injection must have a standardized name. The naming convention must enable the users to quickly understand its purpose. Conformance to a naming convention makes review of data move more quickly-after a convention is in use, nonstandard names are easily seen in a list of sample identifications.
4. Steps to ensure a complete record for review and release: To avoid testing into compliance, the procedure must require personnel to include all injections made while testing, whether they are used to calculate a reportable value or not. Documentation must provide a discussion of data included-and excluded-and the rationale for excluded values. These possible excluded data include aborted runs, runs that fail to meet suitability or method acceptance criteria, excluded replicate values or reinjections of any solution (standard, sample, control, and so forth). In addition, a complete record requires behaviors that create transparency of actions. For example, injections should be labeled as standards, samples, wash, and so forth, before initiating the run. Peak names should be included in the processing method. These actions create an initial record, so changes made postinjection will create an audit trail entry. Labeling injections and peaks postanalysis provides an opportunity for analysts to mislead reviewers by labeling undesired injections or peaks to hide their true content and intent.
5. A process to manage failed (or aborted) runs: Personnel need to have clear understanding of the conditions that permit a set of injections to be stopped or excluded from further use. This process includes documentation within the system, or in other official documentation that provides justification for the exclusion. There must be a means to list the excluded injections in the final review package, because the excluded data must be reviewed for scientific validity before releasing the reportable results.
6. A process to manage extra injections: In this context, extra injections (as defined in your procedure) are any injections of material for chromatographic analysis that are not explicitly addressed in the written method. A classic example is the injection of a reference standard solution (often the middle standard in a series) to assess the response of the system before initiation of system suitability injections. The suitability injections are specified in the analytical method-but the injection before suitability is not authorized in the analytical method or any other reference document (for example, USP monograph).
7. A process to manage manual adjustments to chromatographs or calculations: All manual adjustments from integration parameters to manual baselines, renaming of peaks, processing calculations and external factors included in reported results, must be transparent to the reviewer and included as part of the test records. In addition, the procedure should describe a risk-based review of manual adjustments. For example, all manual adjustments might require review by a senior scientist with extensive assay experience-someone who is able to assess the scientific merit of the integrations and modified calculations.
Review of an Analytical Run
With the basics under control as previously described, we turn our attention to the integration and interpretation of an analytical run. Here, all samples have been injected correctly and the chromatography separations appear to be acceptable. Now look at the individual chromatograms to see if the integration is correct:
- Evaluate in sequence: system suitability test injections, standards, controls, samples. If any of these items fail acceptance criteria, stop!
- Do not review anything involving samples until assay criteria are met.
- Looking at sample results before accepting the run could be considered “testing into compliance,” as if you wanted to invalidate testing you didn’t like (or accept, once you did). Fresenius Kabi Oncology received a warning letter in December 2017 for such behavior (11).
Falsification Practices 1: Peak Shaving and Enhancing
Chromatography is a comparative technique-it compares the response of known standards with unknown samples. It is imperative that standards and samples are processed consistently; otherwise, there will not be a linear relationship between absorption and concentration (Beer-Lambert law). Consistency in peak processing is a strength in automated integration, and one of many reasons for adopting it.
When integrating chromatograms, it is important that chromatographers are aware of bad integration practices used to falsify data (3,8):
- Skimming is a technique where the baselines are manually repositioned to reduce the peak area (see line 2 in Figure 2, where line 1 is automatic peak placement). If performed to the standards, it can increase the amount of analyte in the standards and vice versa.
- Enhancing is the opposite technique, where the peak area is increased for either the standards or the samples to obtain the desired result (see line 3 in Figure 2).
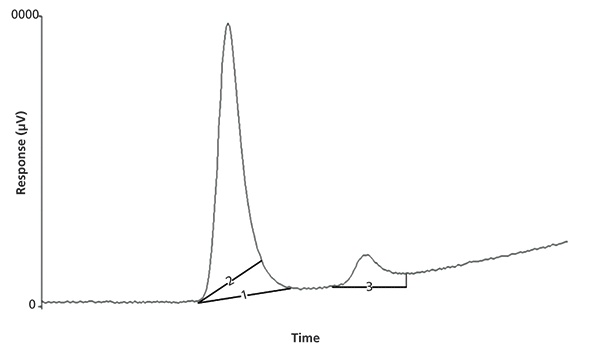
Figure 2: Peak skimming and enhancement.
These two practices must be eliminated in all regulated laboratories.
Keeping these out of integrations can be a challenge for biologics that have large numbers of peaks, often broad, because of their natural origins. The first step in controlling these products is admitting their technical challenges-and therefore their risks-and devoting sufficient resources to method development to automate them. It is a tall order to expect consistency from an analyst who spends 4–8 h manually integrating peaks from a single assay, especially when proper assay development could reduce this task to as little as one hour of labor. Dyson (6) recommended that method-related issues be resolved by additional method development rather than chromatographic processing.
Inhibiting Integration
It is difficult to imagine that anyone involved in the manufacture of pharmaceuticals would consider inhibition of peak areas as a good idea, but a warning letter issued to Divi’s Laboratories in 2017 lists this approach as a practice in the firm (5).
Failure to ensure that test procedures are scientifically sound and appropriate to ensure that your API conform to established standards of quality and/or purity.
Our investigators observed that the software you use to conduct high performance liquid chromatography (HPLC) analyses of API for unknown impurities is configured to permit extensive use of the “inhibit integration” function without scientific justification.
For example, our investigator reviewed the integration parameters you used for HPLC identification of impurities in release testing for [redacted]. These parameters demonstrated that your software was set to inhibit peak integration at four different time periods throughout the analysis. Similarly, in the impurities release testing you performed for [redacted], your HPLC parameters were set to inhibit integration at four different time periods throughout the analysis.
You have been warned: Inhibiting integration is hiding data from review, just as surely as placing printed batch records in a waste can. There can be circumstances where inhibiting is reasonable, such as with solvent fronts or early baseline instability; these situations will be documented in the validated analytical method. Because of the data integrity risks associated with inhibiting integration, its use should be restricted to validated circumstances within your chromatography SOP (above) and training materials.
Integrating Samples First
One foundation of a robust chromatographic SOP requires that samples be integrated and reviewed last. When analysts are permitted to review sample results first, there is the temptation to find a way to reject (or abandon) the entire run because it is now obvious that an undesired test result will be the outcome. To ensure scientific validity, system suitability should be the first set of injections to be processed and compared against acceptability criteria. A system suitability failure invalidates all subsequent injections (12). Assuming the suitability criteria are met, the reference standard injections should be processed and compared against assay acceptability criteria. Only if all criteria are met should other injections be processed, then samples. Processing samples last ensures that good scientific judgment is driving the process rather than testing samples into compliance.
Linking New Samples to Prior Standard and Suitability Injections
Although it is a common practice to assay additional samples over some time period-say, a work shift-and use the initial set of suitability injections and reference standards as the basis of result calculations, there is a data integrity risk that must be considered along with this practice (in addition to proof that the method remains accurate over the time period): testing into compliance. If an undesired sample result appears, an analyst can reinject the sample preparation again (and again), reference the initial standards to obtain a result, and report the injection that provides the desired test result. This behavior is detectable with automated searches that look for test runs with few injections-unless you routinely use short runs for samples arriving through the work shift. As a result, the risk–benefit of routinely using short runs should be weighed.
Is Management the Problem?
Outside of examples such as Leiner Health Products (4), where analysts and supervisors were aware of data manipulation practices, most laboratories create data integrity issues through bad practices, combined with a lack of training. Management is responsible for creating a robust chromatographic procedure, and foundational policies that accompany it. These must be incorporated into training that is received by every analyst to assure consistency in practice. Regulators will cite firms failing to provide adequate procedures covering this critical area of the chemistry quality control (QC) laboratory (7).
In addition to procedures and training, management must require metrics that permit both scientific and quality assurance (QA) personnel to monitor the laboratory data for troubling trends:
- The use of short test runs to assay a sample until it meets a specification (that is, testing into compliance)
- The percentage of peaks integrated automatically and manually for each method and laboratory site
- Assays where samples were integrated before standards based on integration date and time stamps
Trending reports such as these permit oversight of routine processes by pointing to trends that merit additional investigation in an efficient manner.
Management taking the short view costs the firm a considerable amount of money each time injections are manually integrated. Time invested to develop automated integration algorithms returns its cost many times over throughout the life of a method and is a solid investment when a long-term view is considered. In addition to time savings, automation provides consistency from analyst to analyst and run to run. This approach is a clear win for the laboratory from bottom to top.
Summary
We have covered integration of chromatograms in this part of data integrity in the regulated chromatography laboratory. To succeed, a robust SOP for chromatographic integration is essential in today’s environment. Investment in generating robust analytical procedures where automatic integration is the norm is a big win in time savings and consistency. Also, bad integration practices must be identified and eliminated.
In the next part of this series, we will look at the calculation of reportable results.
References
(1) M.E. Newton and R.D. McDowall, LCGC North Am. 36(1), 46–51 (2018).
(2) M.E.Newton and R.D. McDowall, LCGC North Am. 36(4), 270–274 (2018).
(3) R.D. McDowall, Validation of Chromatography Data Systems: Ensuring Data Integrity, Meeting Business and Regulatory Requirements, 2nd Edition (Royal Society of Chemistry, Cambridge, UK, 2017).
(4) FDA Warning Letter to Leiner Health laboratories (U.S. Food and Drug Administration, Rockville, Maryland, 2017).
(5) FDA Warning Letter to Divi’s Laboratories Ltd (Unit II) 2017, available at: https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2017/ucm554576.htm.
(6) N. Dyson, Chromatographic Integration Methods, 2nd Edition (Royal Society of Chemistry, Cambridge, UK, 1998).
(7) WHO Notice of Concern Micro Laboratories, May 2014, available at http://apps.who.int/prequal/info_applicants/NOC/MicroLabs_NoC_30May2014.pdf.
(8) R.D. McDowall, LCGC Europe 28(6), 336–342 (2015).
(9) FDA Warning Letter to Apotex Research Pvt Ltd. 2015, available at: http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/ucm432709.htm.
(10) FDA Warning Letter to Fresenius Kabi Oncology (WL: 320-13-20), U.S. Food and Drug Administration, Silver Springs, Maryland (2013).
(11) FDA Warning Letter to Fresenius Kabi Oncology, 2017, available at: https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2017/ucm589941.htm.
(12) FDA Guidance for Industry Out of Specification Results (U.S. Food and Drug Administration, Rockville, Maryland, 2006).
Mark E. Newton is the principal at Heartland QA in Lebanon, Indiana. Direct correspondence to: mark@heartlandQA.com. R.D. McDowall is the Director of RD McDowall Limited in the UK. Direct correspondence to: rdmcdowall@btconnect.com

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
Investigating the Protective Effects of Frankincense Oil on Wound Healing with GC–MS
April 2nd 2025Frankincense essential oil is known for its anti-inflammatory, antioxidant, and therapeutic properties. A recent study investigated the protective effects of the oil in an excision wound model in rats, focusing on oxidative stress reduction, inflammatory cytokine modulation, and caspase-3 regulation; chemical composition of the oil was analyzed using gas chromatography–mass spectrometry (GC–MS).