Current Progress in Chiral Stationary Phase Development and an Update of Chiral Applications
Special Issues
This discusses using generic chiral stationary phase screening to find the optimum column, smaller diameter columns for HPLC and SFC, clones of off-patent phases, and new concepts in CSP development.
The chiral business continues to grow, albeit at different rates in different parts of the globe. Standard high performance liquid chromatography (HPLC) still dominates the methodologies because of its perceived robustness, transferability, and wide instrument availability. The primary goal of speed and efficiency has been demonstrated through the application of supercritical fluid chromatography (SFC) and columns of smaller dimensions with high solvent flow rates. A handful of successful chiral stationary phases developed over the last several years have prompted a reliance on a generic approach to screening for selectivity. Several instruments introduced to support chiral screening and detection have also furthered the goal of achieving speed and efficiency

Thomas E. Beesley
A number of new clones of the popular cellulose and amylose derivatives have entered the field with a special emphasis on a 3-chloro-5-methylphenyl carbamate derivative of both cellulose and amylose. Two new concepts in chiral stationary phase development have been introduced, one based upon bonded derivatized cyclofructans and the second on a derivatized spiral chiral polymer. In this review, I would like to cover each of these aspects to reflect what has occurred in industry since the time of the last chiral review in the LCGC supplement of April 2008 (1).
The success of chiral separations is highly dependent upon several factors that include the availability and performance of the appropriate chiral stationary phases, the knowledge base of the operator and the methods used to obtain results including the instrumentation. Continued discoveries of significant differences in effectiveness and toxicity of individual enantiomers in biological systems has kept the emphasis on such chiral separations. In addition, the global governmental rules for dealing with compounds that have stereogenic centers has put in place a mechanism for the need for rapid advancement of chiral technology. As a result of these factors, the chiral knowledge base has grown exponentially. Developments in broadly applicable chiral stationary phases (CSPs) in the last several years and continued expansion of the applications data base appears to have created a more routine analytical methodology aided also by appropriate instrument development.
The currently available classes of CSPs have offered more generic approaches, though there are still certain structural types that require new concepts in CSP development. For instance, compounds with stereogenic centers that are nonpolar offering no hydrogen bonding or interactive groups and a significant number of chiral primary amines have been difficult to separate by HPLC. In addition, multiple chiral centers offer unique challenges both analytically and preparatively. On the plus side, advances in screening technologies (that is, use of SFC combined with mass spectrometry [MS], multiple column switching, and parallel column screening systems) has offered better and faster outcomes, a major focus and goal of the pharmaceutical industry.
Numerous publications on a range of chiral screening strategies and their evaluation (2,3) and new insights into mechanisms (4) and molecular modeling (5) have all helped accelerate the advances in the chromatographic separation of stereoisomers. The cited papers represent only a small sample of the many publications available. The first book dedicated solely to the mechanisms of various CSPs, edited by Alain Berthod, will be published this summer by Springer. While the emphases on single enantiomer production has remained intact as a strategy, the pharmaceutical industry in the U.S. has now placed a greater emphasis on proteomics and biologics while the small molecule effort has seen a major shift to Europe and Asia. Europe has seen a significant increase in chiral analysis. China is making a major effort to take its long history of effective natural products and bring their production into 21st century development, typically requiring chiral evaluations. China and India are only now in the early stages of developing the necessary expertise with India showing a somewhat more advanced progress in chiral analysis. Even though the apparent numbers of chiral compounds in the U.S. pipeline has not increased significantly, the stress on analytical chemists to come up with chiral methods quickly and efficiently has not diminished. Little time or effort is given to optimizing a separation or searching for the optimum CSP. Speed and efficiency remain the targets. This seems like a flawed strategy when method redevelopment has to be done or when the needs of the method changes (that is, requiring a change in mobile phase type from normal phase to reversed phase in manufacturing processes or polar organic to SFC for preparative chromatography needs). Aspects of drug development require changes to tolerable mobile phase components.
The State of Chiral Screening Strategies
The success of chiral methods development often is complemented by the introduction of appropriate instrumentation. This includes everything from column heaters–coolers, a very important operating parameter in chiral chromatography to screening and detection systems such as optical rotation (LC–OR), circular dichroism (LC–CD), and mass spectometry (LC–MS).
Instrumentation specifically development for multicolumn screening will make selecting the optimum CSP much easier. For example, the Express LC systems (Eksigent, Dublin, California), recently acquired by AB Sciex (Foster City, California) offer an eight-microcolumn screening system that claims to reduce method development time by factors of 10 with 1/10 the solvent consumption. Sepiatec GmbH (Berlin, Germany) also offers an eight parallel column HPLC screening system trademarked Sepmatix, as well as a similar eight-column system for SFC.
In the last coverage of this subject on chiral separations (1), a number of approaches were being investigated including sequential screening of four to as many as 12 CSPs under an established protocol, and the introduction of a number of parallel screening systems as well as packed capillary screening systems described in the previous review. Statistics are not available but from a brief survey of users in the field, these options appear to have now settled down in large part to a screen of four or eight chiral stationary phases with backup schemes when the first pass-through fails. A number of recent publications also have introduced the concept of using an SFC tandem column screening system (6,7), a growing trend. In one case (6), a two-by-four column system was operated in parallel, claiming for five sequential samples that it took 29 min on average for the method development of each sample. Screening of each four-channel column typically took 5 min and each optimization took ~15 min. Of the more than 100 samples processed by this method, the authors claimed remarkable success. The system comprised two banks of four columns (150 mm × 2.1 mm) that included ChiralPak AD-H, AS-H, ChiralCel OD-H, OJ-H (all available from Chiral Technologies, West Chester, Pennsylvania) and the Chirobiotics V, T, R, and TAG (products of Sigma Aldrich/Supelco, St. Louis, Missouri).
To further expand this generic screening activity, PDR-Chiral (Lake Park, Florida), has developed a 12- or 24-column automated method-screening system with a 10- or 20-bottle solvent mixer. The system can be operated as an HPLC or SFC system purchased with or without heating and cooling and is equipped with both UV and laser polarimeter detectors to identify chiral components and elution order. In a recent paper, four currently available HPLC chiral detectors, three polarimeters, and a CD detector were evaluated for their performance in chiral screening systems (8). The CD detector was considered the most useful as its response is both linear and sensitive except for non-UV analytes, for which the polarimeter offers a distinct advantage.
The battle for fast chiral analysis has taken many paths to date looking for the optimum solution. In the absence of sub-2-μm CSPs availability, a recent paper demonstrated the effectiveness of ultrahigh-pressure LC (UHPLC) for fast chiral separation of drugs by first derivatizing the analytes: rac amphetamine, rac methamphetamine, and several β-blockers with AITC and Marfey's reagent respectively. The separation of the resulting diastereoisomers was run on the sub-2-μm Acquity C18 column (Waters Corp., Milford, Massachusetts), resulting in the separation of up to 10 derivatized enantiomers in less than 3 min (9). While this can be a useful methodology for pure substances, it presents its own analytical challenges, especially for the clinical analysis of amines.
The State of Current CSPs
Of the over 110 CSPs that have been introduced in the last 10 years, there are currently a handful that have appeared to satisfy the majority of the chiral separation needs of the industry. The derivatized cellulose and amylose phases still dominate the chiral market, while the macrocyclic glycopeptides applications have expanded significantly and taken on a complimentary role. Cyclodextrin derivatives, like in the CYCLOBOND line (Sigma Aldrich/Supelco), and π-complex CSPs like Whelk-01 (Regis Technologies, Morton Grove, Illinois) have largely fulfilled the balance of the needs, the choice depending upon compound structure and the particular mobile phase requirements of the application. In some instances, as in the area of clinical applications, the immobilized protein phases can be added to the preceding list. From a review of the literature, many of these latter applications outperformed the popular cellulose- and amylose-based phases based upon sample solubility due to mobile phase composition, selectivity and separation of multiple chiral centers, or degradation products within the sample.
Polysaccharide Chiral Stationary Phases
At the time of the last review, Chiral Technologies, Inc. (Daicel), had just introduced CHIRALPAK IC, a bonded 3,5-dichlorophenylcarbamate derivative of cellulose. This phase had never been made available as a coated version due to its high solubility in organic solvents. As a bonded column, it has demonstrated good selectivity for those analytes previously not resolved or poorly resolved by the other immobilized cellulose and amylose derivatives. This column is now considered to be complementary to the other two bonded phases. More complex method development protocols and the expanded use of high levels of methyl tert-butyl ether (MTBE) with this phase, however, limits its applicability in certain applications. Chiral Technologies also has added two new 5-μm coated derivatives to its line, the methylchlorophenyl carbamate of cellulose (CHIRALPAK AY-H) and of amylose (CHIRALCEL OZ-H). These phases originally were available coated on 20-μm silica particles for preparative applications only. Figures 1 and 2 demonstrate a comparison to the original, more standard dimethylphenyl carbamate derivatives of cellulose (OD) and amylose (AD), demonstrating enhanced selectivity. In addition, they have extended the CHIRALCEL OD and CHIRALPAK AD stationary phases to 3-μm material for LC–MS applications. A 50 mm × 4.6 mm column producing 7000 plates offers high speed analysis with manageable pressures. Stability testing up to 250 bar (3625 psi) showed no deterioration in aqueous mobile phase systems.
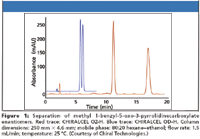
Figure 1
Macrocyclic Glycopeptide Phases
An HPLC method development strategy and applications update had been published in 2009 (10) for the macrocyclic glycopeptides (CHIROBIOTIC) phases (Supelco/Sigma Aldrich). The article cites their unique breadth and versatility in a variety of mobile phase conditions suitable for clinical applications using LC–MS methods, drug discovery screening, and preparative methods. The article identifies the potential mechanisms at work and the mobile phase that drives those specific interactions. For these and other phases in their line, a new search engine has been developed (Supelco/Sigma Aldrich) for obtaining chromatographic information on a particular chiral analyte of interest, compound class, or applications on a particular chiral column. Ideas, tips, and tricks for chiral chromatographers also are offered on Twitter. For more information on this latest innovation in chiral communication, go to www.twitter.com/chiralchrom.
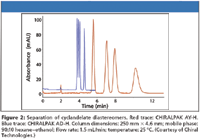
Figure 2
Cyclodextrin-Based Phases
Cyclodextrin technology has a long history of development dating back to the 1970's. It is ubiquitous in its breadth of applications both for chiral and achiral analytes. It continues to find its mark in current chiral processing. A surprise has been the unique opportunities it has afforded in SFC (11) for the π-acidic derivative CYCLOBOND DNP (Supelco). Another unexpected discovery was the ability of the CYCLOBOND DM phase, a fully methylated version with no hydrogen bonding capabilities, demonstrating good selectivity for single ring structures with bulky side arms and fused ring structures with a dominance of steric interactions as the driving force (12–14).
π-Complex Based Phases
With the increasing interest in SFC, both for analytical and preparative applications, the π-complex phases have seen an increase in activity due primarily to their success in normal-phase conditions. The Whelk-01 and -02 phases have been the workhorses in this area. A recent application note by Regis Technologies demonstrated the effect of varying cosolvents in SFC on Whelk-01. It showed that an increase in polarity of the modifier correlated with a decrease in selectivity and a decrease in retention (Figure 3).
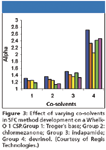
Figure 3
Preparative Applications
While SFC has taken a very substantial role in the chiral business, so far, it has been employed primarily, though not exclusively, to preparative applications for all its green benefits as well as for its speed. Comments from analysts in the field indicated that the major reason that HPLC still seems to be the method of choice is the robustness, transferability, and wide instrument availability of standard HPLC equipment. The rule seems to be that methods based upon typical normal-phase solvents or from the polar organic mode to a lesser degree can be transferred to SFC, while those developed with a reversed-phase system were less successful. In addition to speed and the reduction in solvent consumption, it has reduced the solvent waste stream substantially. Development of a fraction collector for the in-line concentration of purified enantiomers by Modular SFC, Inc. (North Attleboro, Massachusetts) certainly will further its utility and cost effectiveness.
There are still a significant number of applications requiring conventional HPLC, and the recent introduction of a versatile benchtop simulated moving bed (SMB) system called Semba Octave, (Semba Biosciences, Madison, Wisconsin) should help bridge the gap in cost effectiveness. Supelco (Bellefonte, Pennsylvania) was a test site for this device and now has introduced a set of eight 15-μm particle size, 10 mm × 5 mm columns designed to operate with this system or any benchtop HPLC system. (Figure 4)
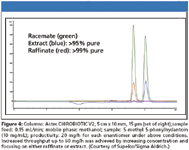
Figure 4
The Year of the Clones
With expiration of the Chiral Technologies' (Daicel) patents on their highly successful coated versions of the derivatized cellulose and amylose phases, an increasing number of companies have attempted to produce generic versions with varying degrees of success.
The successful Kromasil entries AmyCoat a 3,5-dimethylphenyl carbamate derivative of amylose and CelluCoat the 3,5-dimethylphenylcarbamate derivative of cellulose from Akzo Nobel (Bohus, Sweden), have announced an agreement with Sigma Aldrich/Supelco for exclusive distribution in the U.S., Canada, and Puerto Rico. In poster 17P at the 2010 Pittcon, Eka ChemicalAB demonstrated the optimization of small-scale chiral HPLC preparative separation of tropic acid on a Knauer benchtop SMB system (Berlin, Germany) using eight 150 mm × 4.6 mm (25 μm) Amy Coat columns. Total development time was said to be two working days with the Knauer SMB-Guide software.
Phenomenex (Torrance, California) in this period introduced the Lux chiral line that includes a 5-chloro-2-methylphenyl carbamate derivative of both cellulose (Lux Cellulose-2) and amylose (Lux Amylose-2). There is also as part of this line a replacement claimed to match the CHIRALCEL OD-H CSP from Chiral Technologies referred to as Lux Cellulose-1.
ES Industries (West Berlin, New Jersey) also has introduced Chromega Chiral CC2, a 3-chloro-5-methylphenyl carbamate derivative of cellulose coated on a 5-μm spherical 1000-Å silica that they claim has improved efficiency.

Table I: Examples of racemic separations on Epitomize CSPs
Orochem Technologies (Lombard, Illinois) now has introduced four products in this area with a trade name of Epitomize. This line of phases includes the typical 3,5-dimethylphenyl carbamate derivatives of both cellulose (CSP-1C) and amylose (CSP-1A) but an interesting 3-chloro-4-methylphenyl carbamate derivative again of both cellulose (CSP-1Z) and amylose (CSP-1K). For a comparative enantiomeric response see Table I. In addition to the usual variety of particle sizes and column lengths, Orochem has added a UHPLC version for LC–MS utilizing 1.7-μm particles (Figure 5). This format is available for all four coated versions with a pressure limit of ~3500 psi.
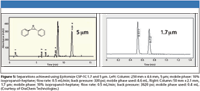
Figure 5
New Chiral Stationary Phases
A number of the earlier attempts to introduce new concepts in CSPs have all but disappeared including chiral molecular imprinting. Chiral ionic liquids have been developed for a wide variety of achiral applications including their recent successful application in the chiral area for gas chromatography (15) and HPLC (16). One of the consistent failures in the chiral separation business has been the separation of a significant number of primary amines. The crown ethers do a very good job in separating many of these types of compounds but not all and they fail to deliver on preparative, clinical, and some manufacturing processes because of the lack of mobile phase volatility.
Dr. Daniel Armstrong (University of Texas, Arlington) has now published on a new series of bonded CSPs based upon derivatized cyclofructans and appears to have more than solved the problem (17). Three products in this series will be launched this year. The first is based upon an isopropyl derivatized C6 cyclofructan (CF6) that efficiently separates chiral primary amines. Second, a highly aromatic derivatized C6 cyclofructan that loses its ability to separate these chiral amines but has excellent selectivity towards a broad spectrum of other types of racemates is in beta testing. A third product, also highly derivatized with a different aromatic functionality is based upon a cyclofructan-7 (CF7) and offers complimentary selectivity to the other aromatic derivatized CF6 CSP for different classes of racemates. The interesting fact is that the dominant mode of separation for all of these phases is either normal phase or polar organic mode, both of which allow for direct transfer to efficient SFC methodologies (Figure 6). With the isopropyl derivative on the CF6, over 20 chiral primary amines were separated to baseline in one day. Stability was addressed, indicating no change in performance in all common organic solvents even after over 1000 injections. These stationary phases also seem to have good sample capacity as demonstrated by the 4200 μg in 100 μL resolved to baseline on an analytical column, in this case limited only by the sample solubility.
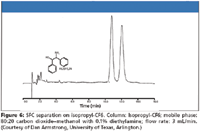
Figure 6
Toyko Kasei (TCI, Tokyo, Japan) has introduced a new concept in chiral stationary phase development with a spiral chiral polymer that offers steric recognition as the primary mechanism. Three different products result when chiral side groups are attached to the backbone, adding additional mechanisms. The three offer, in addition to steric recognition from the side chain, electrostatic interactions between an analyte containing a carbonyl group and a nitrogen side chain and π-π interaction between an analyte and the side chain. The polarity of the three products ranges from low for the TCI Chiral MB-S to moderate for the TCI Chiral BP-S to high polarity for the TCI Chiral CH-S, covering a broad range of chiral applications in either normal or reversed-phase conditions. The products come in 3- and 5-μm formats.
A new approach for biomedical analysis of chiral compounds was demonstrated with the direct separation and quantification of lactic acid enantiomers in human urine. This was reported using for the first time a high-performance immunoaffinity LC–MS system. The antibody was immobilized onto a POROS-OH 20-μm support material from Perceptive Biosystems (Cambridge, Massachusetts), activated and packed into a 150 mm × 4.6 mm stainless-steel column. Pure alpha hydroxyl acids were injected and eluted with a buffer, demonstrating the selective binding of the D-enantiomer but not the L. It was determined that the limit of detection when binding the D-hydroxyacid antibody for lactic acid enantiomers using MS as a detector was 63 μM (18).
Conclusions
Chiral analysis continues to expand and to gain in speed and efficiency, driven by the use of SFC and small column design at high linear velocity. Generic screening for selectivity remains the method of choice with four to eight columns operated either sequentially or in parallel with the availability of new screening instrumentation. New versions of the popular coated cellulose and amylose derivatives continue to enter the market. Two new concepts in chiral stationary phase development have been introduced based upon a bonded and derivatized six-carbon or seven-carbon cyclofructan and a derivatized spiral chiral polymer.
Thomas E. Beesley Founder and former CEO, Quantum Industries, manufacturer TLC. Designed pre-adsorbent and channeled TLC. Sold to Whatmann 1978. Founder & CEO, Advanced Separation Technologies Inc, manufacturer chiral HPLC; Cyclobond & Chirobiotic lines and chiral GC; Chiraldex phases. Sold to Sigma-Aldrich 2006. Currently lecturer, expert witness, contract problem solver with speciality in separation of chiral molecules.
References
(1) T.E. Beesley, LCGC Supplement: Recent Developments in Column Technology 26(S4), 43–46 (2008).
(2) H.A. Wetli and E. Francotte, J.Sep. Sci. 30, 1255–1261 (2007).
(3) B.L. He and Y. Shi, Am. Pharm. Res. 11, 47–52 (2008).
(4) C.R Mitchell, N.J. Benz, and S. Zhang, J. Chromatogr., B 875, 65–71 (2008).
(5) A. Berthod, S.C. Chang, and D.W. Armstrong, Anal.Chem. 64, 395–404 (1992).
(6) L. Zeng, R. Xu, D.B. Laskar, and D.B. Kassel, J. Chromatogr. A 1169, 193–204 (2007).
(7) C.J. Welch, M. Biba, J.R. Gouker G. Kath, P. Augustine, and P. Hosek, Chirality 19, 184–189 (2007).
(8) L. Kott, B.W. Holzheuer, M.M. Wong, and G.K. Webster, J. Pharma. Biomed. Anal. 43, 57–65 (2007).
(9) D. Guillarme, G. Bonvin, F. Badoud J. Schappler, S. Rudaz, and V. Jean-Luc, Chirality 22, 320–330 (2010).
(10) T.E. Beesley and J.T. Lee, J. Liquid. Chromatogr Rel. Technol. 32, 1733–1767 (2009).
(11) K.H. Gahm, Poster 78 of the 18th International Conference on Chirality, ISCD- 18, Busan, S.Korea, June 24–28 (2006).
(12) X. Han, Q. Huang, J. Ding, R.C. Laroch, and D.W. Armstrong, Sep. Sci. Tech. 40(13), 2745–2759 (2005).
(13) X. Han, T. Yao, Y. Liu, R.C. Laroch and D.W. Armstrong, J. Chromatogr., A 1063, 111–120 (2005).
(14) D.D. Schumacher, C.R. Mitchell, R.V. Rozhkev, R.C. Laroch, and D.W. Armstrong, J. Liquid Chromatogr. Rel. Tech. 28(2), 169–186 (2005).
(15) J. Ding, T. Welton, and D.W. Armstrong , Anal. Chem. 76, 6819–6822 (2004).
(16) S. Yu, S. Lindeman, and C.D. Tran, J. Org. Chem. 73, 2576–2591 (2008).
(17) P. Sun, C. Wang, Z.S. Breitbach, Y. Zhang Y., and D.W. Armstrong, Anal. Chem. 81, 10215–10226 (2009).
(18) E.J. Franco, H. Hofstetter, and O. Hofstetter, J. Pharm. Biomed. Anal. 49, 1088–1091 (2009).

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









