Column Triage
LCGC North America
How do you react to symptoms that indicate your liquid chromatography column has major problems?
With the popularity of medical shows on television, you'd think we'd all know how to act in a medical emergency. After all, we know what to do when we have an emergency with our liquid chromatography (LC) column, don't we? Or is it really an emergency? In this month's "LC Troubleshooting," we'll consider the process of column triage. When will the column survive on its own? When does it have recoverable injuries? And when is it beyond resuscitation? We'll concentrate on three primary symptoms of column failure: increased peak tailing, increased pressure, and changed retention times.
"Good Enough" May Be Trying to Tell You Something
A well-designed LC method should have a system suitability test that truly evaluates the ability of the method to perform. Usually the system suitability test includes a measure of peak tailing (tailing factor, TF, or asymmetry factor, As) and a retention time, tR, or retention-time window for the peak or peaks of interest. Often, resolution, Rs, is included. I also suggest that system pressure should be noted on a daily basis. If your LC system doesn't record the pressure automatically, add an item to the run summary that usually is part of each method. Rarely is a change in pressure alone a predictor of poor data quality, but it is one of the early warning signs that the column may encounter problems.
When one or more of the system suitability parameters fails to meet the pre-defined limits, something must be done to correct the problem, such as mixing a new batch of mobile phase or cleaning or replacing the column. However, if this is the only way system suitability results are used — as a a pass–fail decision — you are not taking advantage of the information that is available. You may find system suitability failure comes as a surprise: One day everything is OK, then the next day it fails. This is rarely the case, however; instead, the system often gives hints of what is happening, if you pay close attention. I recommend making a control chart to follow the trend of each of the system-suitability parameters, and I would track pressure in the same way. The control chart is simply a plot of the value of the parameter over time or number of injections, with the acceptable limit of the parameter plotted as well. You can be fancy and add more traditional control-chart features, such as those described in (1), but that may be overkill for what you need for system suitability.
I have created three control charts in Figure 1 to illustrate the point. These are data for a fictitious method over 30 batches of samples. In the top panel, the pressure at the beginning of the run is plotted (solid line) with a dashed line added at a 2500-psi boundary. Normally the method runs in the 1500–2000 psi range, and when it gets to ≈2500 psi it is time to change the frit in the in-line filter. This 2500-psi boundary is a "soft" limit, because data quality is seldom compromised if the pressure exceeds this value. The middle panel plots the tailing factor, with a system suitability requirement that TF should be ≤1.6 (dashed line). With a new column, TF ≈ 1.4 is observed. The lower panel shows the retention time during system suitability with a normal value of ≈4.7 min and a requirement of 4.5 ≤ tR ≤ 4.9.
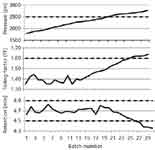
Figure 1: Example control charts to trend column performance. Pressure (top), tailing factor (middle), and retention (bottom) are plotted as solid lines over 30 runs with method limits shown as dashed lines.
If we blindly use the system suitability data of Figure 1 to tell us whether the system is "good enough" to make it though the next batch of samples, we may be surprised when system suitability fails. Instead, think of how these data are trying to give us early warning signs. For example, you can see that variation in retention and tailing seem fairly random until approximately batch 20, when they begin trending toward the limits. It looks like the limits are reached for both these parameters after ≈25 batches. If column replacement reset the system suitability parameters to where they started, and similar trends were observed for additional columns, these data could be used to establish a preventive maintenance strategy. For example, proactive replacement of the column after 20 sample batches would avoid any problems created by system suitability failure, and excessive pressure would be avoided, too. My recommendation is that if you can anticipate when a part (the column in the present example) will fail, replacement of the part at 70–80% of its expected lifetime is a good strategy. You get most of the useful life out of the part and avoid any problems associated with a failure of that part.
Crash-Cart Time
So what happens when system suitability fails? The three most common symptoms that something is not right with the column are increased pressure, increased tailing, and a change in retention times, either individually or in combination. The causes of column problems are physical or chemical in nature. Let's look at each of these.
Physical problems with the column most commonly are manifested as a build-up of particulate matter on the inlet frit of the column. This will cause increased pressure and often similar peak distortion of all the peaks in the chromatogram. For example, all peaks will tail excessively or may be split or doubled. When these symptoms are observed, you know that whatever happened occurred before the sample was separated. About a third of the time you can rescue a column exhibiting these symptoms by reversing it and back-flushing it. Simply remove the column, reverse it, and route the new outlet end to waste (not to the detector). Flush the column with 15–20 mL of mobile phase, or better yet, with the strong solvent of the mobile phase (generally acetonitrile or methanol). The hope is that this will wash the particulate matter off the inlet frit and reduce the pressure back to acceptable levels. If the column is packed with 3.5- or 5-µm silica-based particles, you can leave the column reversed. Reconnect it to the detector and check system suitability again to see if it will pass now. Columns packed with particles ≤3 µm in diameter may use inlet frits that are too large to allow you to continue operation of the column in the reversed direction, so you should return the column to its original direction in such cases. It is a good idea to consult the column care-and-use instructions to see if there are any limitations on column reversal. If you are lucky, this procedure will revitalize the column and you'll be able to use it a bit longer.
Chemical problems with the column usually show up as changes in retention for one or more peaks, as increased peak tailing, or as a combination of retention changes and peak tailing. Strongly retained materials from the sample can build up on the column over time, causing a change in the column chemistry, and thus a change in the appearance of the peaks. Sometimes these materials can be removed by flushing the column with a strong solvent. Another cause of retention and tailing changes is that something has attacked the stationary phase or otherwise permanently changed the chemical surface of the column. In such cases, the column cannot be restored. It is not easy to distinguish between the two forms of failure, so the same treatment should be tried to see if it will help. Remove strongly retained material by flushing the column with 10–20 column volumes (15–20 mL) of the strong solvent of the mobile phase. If you are lucky, this will revive the column.
If symptoms of physical or chemical problems with the column occur regularly before 500–1000 injections have been performed, you may be able to extend column life by improving the sample cleanup or adding column protection to the system.Because sample cleanup is more involved, I recommend starting with column protection. Every system should be operated with an in-line filter immediately downstream from the autosampler. For 3- or 5-µm columns, this filter contains a 0.5-µm porosity frit. This will trap particulate matter that would otherwise get caught on the 2.0- or 0.5-µm frit at the head of the column. When the pressure begins to rise, replace the frit. The frit in the in-line filter can be changed in a few minutes and is very inexpensive, especially when compared to the cost of the column. The use of a guard column is another option to help extend column life. The frit at the head of the guard column will trap debris that would otherwise block the column inlet frit, but even this can be avoided if you use the in-line filter in addition to the guard column. Guard columns have been shown to extend the life of the analytical column, but they may cost 20% of the cost of the analytical column, so the economics of guard column use may be marginal. If you use a guard column, you'll have to figure out when to replace the guard column. You should replace the guard column before it adversely affects the overall separation. As with the parameters of Figure 1, track the system performance when a guard column is in place. When system suitability begins to trend toward the limits, change the guard column and see if it fixes the problem. Most workers find that it is more convenient to replace the guard column on a scheduled basis — either after a certain number of injections or sample batches — or perhaps on a calendar basis. Additional sample preparation almost always will extend column life, but sample preparation is expensive and may not be appropriate for all sample types.
Better Luck Next Time
At some point, the simple restorative measures of flushing or backflushing the column will not be successful. As mentioned above, these measures restore column performance only a third of the time. This means that two-thirds of the time they fail. What next? Some people like to flush with even stronger solvents, such as methylene chloride, to remove fats and waxes (be sure to flush the column before and afterwards with methanol or acetonitrile to ensure solvent miscibility). Other flushing strategies use acid, base, or chaotropic agents to remove strongly retained material from the column. If you try any of these procedures, you should backflush the column so the strongly retained material that usually is located on the first few millimeters of the column can be washed directly to waste rather than farther into the column. However, I feel that heroic measures such as these are false economy. They rarely restore column function completely, and they are expensive and time-consuming.
The column should be considered a consumable item, even though the price tag may make us think that it is a capital expense. Consider the cost of the column in relation to the overall cost of analysis. The overall cost of analyzing a sample using UV detection often is in the range of $50/sample. Columns cost ≈$500 each. If the method conditions result in a relatively short column lifetime of 500 injections, that translates into $1/sample for the column, only 2% ($1/$50) of the cost of analysis. If you get >1000 samples through a column, the column is a trivial part of the overall cost of analysis. Any work to extend the column life beyond this may cost more than the additional benefit, because time is an expense. Even backflushing may not be worthwhile. You are better off using an in-line filter to catch unwanted particles from blocking the column and leave it at that. I have a friend that replaces the column with each 96-sample batch — the ≈$5/sample cost of the column is less than the cost of additional sample pretreatment that would extend the column life. It's all about economics. All columns will die eventually.
Summary
The column always tries to tell us how it is doing. Listen to the system suitability results and see if you can find trends that can be used to your advantage. Perhaps you can only anticipate the column failure by a day or two, but you may be able to use the information to put together a preventive maintenance strategy that includes a regular flush with a strong solvent and the use of a guard column to help keep the column in service for months on end. As a minimum, I recommend using an in-line filter on every system and flushing the column with the strong solvent of the mobile phase at the end of each batch of samples. Control charts can be a powerful tool to help anticipate when problems will occur in the future. Above all, remember that the column is a consumable item that may not be worth trying to save after 500–1000 samples have been analyzed.
John W. Dolan "LC Troubleshooting" Editor John Dolan has been writing "LC Troubleshooting" for LCGC for more than 25 years. One of the industry's most respected professionals, John is currently the Vice President of and a principal instructor for LC Resources, Walnut Creek, California. He is also a member of LCGC's editorial advisory board. Direct correspondence about this column via e-mail to John.Dolan@LCResources.com.

John W. Dolan
References
(1) D.J. Wheeler, Understanding Variation. The Key to Managing Chaos (SPC Press, Inc., Knoxville, Tennessee, 1993).

Measuring Vitamin K1 Concentrations in Dogs with Chronic Enteropathy Using LC–MS/MS
May 14th 2025A joint study between the University of Tennessee (Knoxville, Tennessee) and the University of Pennsylvania School of Veterinary Medicine (Philadelphia, Pennsylvania) compared directly measured vitamin K1 (vitK1) concentrations in healthy dogs and dogs with chronic enteropathy (CE) using liquid chromatography tandem mass spectrometry (LC–MS/MS); they also investigated whether supplementation of vitK1 in dogs with CE would significantly increase vitK1 concentrations.
HPLC 2025 Preview: Fundamentally Speaking (Part 2)
May 14th 2025Michael Lämmerhofer from the Institute of Pharmaceutical Sciences, University of Tübingen, Germany, spoke to JFK Huber Lecture Award winner of 2024 Torgny Fornstedt, professor in analytical chemistry and leader of the Fundamental Separation Science Group, Karlstad University, Sweden, about his pioneering work in high performance liquid chromatography (HPLC) with a focus on fundamentals, ion-pair chromatography, and oligonucleotide applications.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









