Class is Back in Session: More Questions on Your Extraction Knowledge (EU)
In our September instalment of “Sample Preparation Perspectives”, we shared sample exam questions from our graduate Chromatography and Separations course as a refresher of some fundamental principles and to give readers the opportunity to test their knowledge. We’ve dusted off our files and found additional questions for this month’s column. These questions tend to focus on dealing with octanol-water partition coefficients, as well as more practical applications of sample preparation.
Question 1: Experimental measurements of Kow are not necessarily straightforward. Describe how you would go about measuring the Kow for a solute.
Kow, or the octanol-water partition coefficient, is widely used to describe the distribution of solutes in biochemical, environmental, and analytical systems between nonpolar (lipophilic) and aqueous phases. It is defined as Kow = [S]oct/[S]aq, though it is not simply the ratio of the solubility of the solute (S) in the octanol and water phases. One must consider all equilibria occurring in the system, including the mutual solubility of the two solvents. Prior to making the determination of Kow, the octanol and water phases must be brought into contact with each other, thoroughly mixed to obtain saturation of each solvent in the other, and allowed to separate into two separate phases in equilibrium. The solute of interest should then be added until saturation is reached, and equilibrium between the octanol and water phases is achieved. Mixing can help obtain equilibrium more quickly. Solute concentration in each phase can be measured via chromatography, spectroscopy, or other suitable means. Specific procedures for measuring Kow can be found in the methods of a number of regulatory and trade organizations.
Question 2: One common method for the extraction of fat from food samples is to use hexane. If you attempt to extract fat from foods using hexane in a microwave-assisted extractor, what will happen? Why?
Only materials with a dipole moment will absorb microwave radiation. As an overly generalized statement, a material’s dipole will align with the electromagnetic radiation and oscillate with the frequency of the radiation. The resulting molecular vibrations release energy, which serves to heat the surrounding material. Hence, hexane, which is nonpolar, will not absorb microwave radiation. In this case, any microwave absorption, and subsequent heating, will occur because of any residual water in the food. If one wishes to perform microwave-assisted extraction (MAE) with nonpolar solvents, there are a couple general ways this can be done. Addition of a polar solvent to the extracting solvents can be used. However, considering the “like dissolves like” principle, convenient systems combining polar and nonpolar solvent are somewhat difficult to obtain. Most modern, commercial microwave-assisted extraction systems have an available “susceptor”, which will absorb the microwave irradiation and pass along the resulting heat via convection.
Question 3: Lipids samples, such as triglycerides, are generally hydrolyzed with an acid, then esterified to form fatty acid methyl esters (FAME) prior to analysis for total fats, as seen in equation 1 below. The FAME are readily analyzed by gas chromatography–flame ionization detection (GC–FID). For example, this method is used for compliance with the Nutritional Labelling and Education Act, specifically in the food content labels we see in the United States.
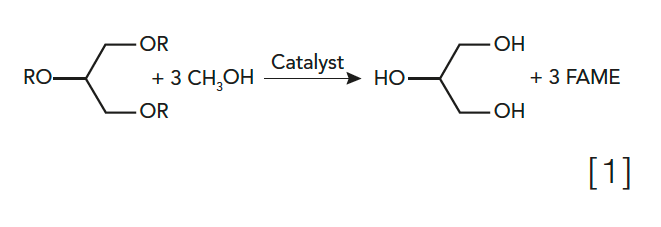
Sucrose esters (see equation 2 below) are approved as fat substitutes when the R group is a fatty acid. Based on the chain length and saturation of the fatty acids used and the degree of esterification, the resulting sucrose ester will mimic a traditional lipid in terms of solubility properties, melting point, viscosity, and surface tension, among other traits.
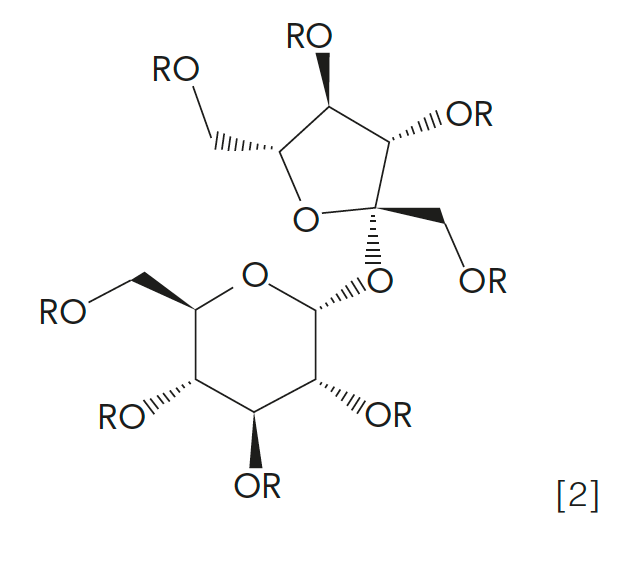
Now, consider the situation of a food product that contains naturally occurring triglycerides, but is also processed with the sucrose ester. If acid hydrolysis and FAME analysis is used in the determination of lipids, the R groups in the sucrose ester would also be acid hydrolyzed, so the presence of the sucrose ester would give an artificially high value (such as a false positive) for the determination of triglycerides (analyzed as FAME). Suggest an extraction method with suitable selectivity to determine triglycerides in the presence of the sucrose ester.
This question can evaluate both an understanding of extraction selectivity and creative application of chemical knowledge. Because the properties of the sucrose ester are similar to traditional lipids, we cannot perform an extraction based on differential solubility. Perhaps the most common answer to this question is to perform some type of extraction which gets the FAME (and lipids) into the solution phase, followed by solid-phase extraction (SPE). This is certainly a promising method and should work, though finding the appropriate combination of solvents and stationary phases is likely to take a great deal of research. Some students will suggest exclusion phases to perform the requisite separation of triglyceride from sucrose ester. Other forms of solvent extraction, such as pressurized solvent extraction (PSE), with the sorbent material added to the extraction vessel, may expedite the extraction, though the added effect of the heat may make obtaining suitable selectivity challenging.
The next most promising method would be supercritical fluid extraction (SFE). By manipulation of the applied temperature and pressure, one should reasonably expect to separate various classes of lipids, including the sucrose esters, from each other.
The method developed by the industrial creator of the sucrose ester (1) relies on the traditional method, with the exception that the hydrolysis step is performed with lipase. These enzymes offer selectivity, as they look for the three-carbon glycerol backbone of mono-, di-, and triglycerides, and will not recognize the sugar backbone of the sucrose ester (the same principle by which the sucrose esters are “fat free”).
To one trained in biochemistry or food science, this approach may seem straightforward. However, to many analysts, this approach requires some critical understanding of the chemistry of the system being studied, as well as out of the box thinking since this type of approach is not found in traditional analytical training.
Question 4: The Environmental Protection Agency (EPA) method 625 (2) is used for the determination of bases, neutrals, and acids in drinking water and wastewater via liquid–liquid extraction (LLE) of 1000 mL of sample. An SPE alternative (3) has been developed that uses 100 mL of sample, significantly less solvent, and gives better analyte recovery. Table 1 provides a method results summary for spiked synthetic wastewater. Discuss the advantages and disadvantages of SPE compared with LLE for this extraction.
For this question, we can assume that the student has not reviewed the application note with the new method or the EPA method, though this assumption may be incorrect for a take-home or similar exam. Based strictly on the information presented in the table (and devoid of other potentially relevant information), evaluation of the sample size indicates that the disk format of SPE must have been used. SPE cartridges are typically used of sample sizes smaller than tens of millilitres. Compared with LLE using samples of similar size (1000 mL), the SPE disk approach is approximately 1.3–1.7 times faster, and uses approximately 45% less solvent. With less solvent use, there is an associated green advantage of less waste, lower flammability hazard, and less use of potentially toxic solvents. Also, less solvent means lower cost and faster solvent evaporation steps. SPE does not deal with emulsion formation problems. Standard deviations are not shown, so we cannot comment on the reproducibility of the methods shown. Whether LLE or SPE is superior in terms of analyte recovery from one-litre samples is unclear. The recoveries shown, lower than the recoveries with SPE of a 100‑mL sample, may be because of the difficulties of handling one-litre samples. However, closer inspection reveals that the recovery may be related to analyte acidity, so use of a mixed-mode SPE phase, perhaps by combining two or more disks, may improve the recovery via SPE. Use of 100-mL samples with SPE demonstrates several interesting features. First, the recoveries are consistently higher across all analyte types, indicating that perhaps the sorbent loading of the disks is more appropriate for the smaller samples. Another consideration is that, with the 100-mL samples and SPE, the ratio of solvent eluent volume to sample amount (0.8) is much larger than with the 1000-mL samples (0.2 for SPE and 0.36 for LLE). This increase in the relative amount of solvent used may explain the further reduction in extraction time and increased recoveries.
Question 5: Figure 1 (4) shows that, everything else being equal, stir-bar sorptive extraction (SBSE) can extract more solute (of equal Kow) than solid-phase microextraction (SPME), and that solutes with a Kow too low to be quantitatively extracted with SPME may be effectively extracted with the SBSE method. Explain why this is.
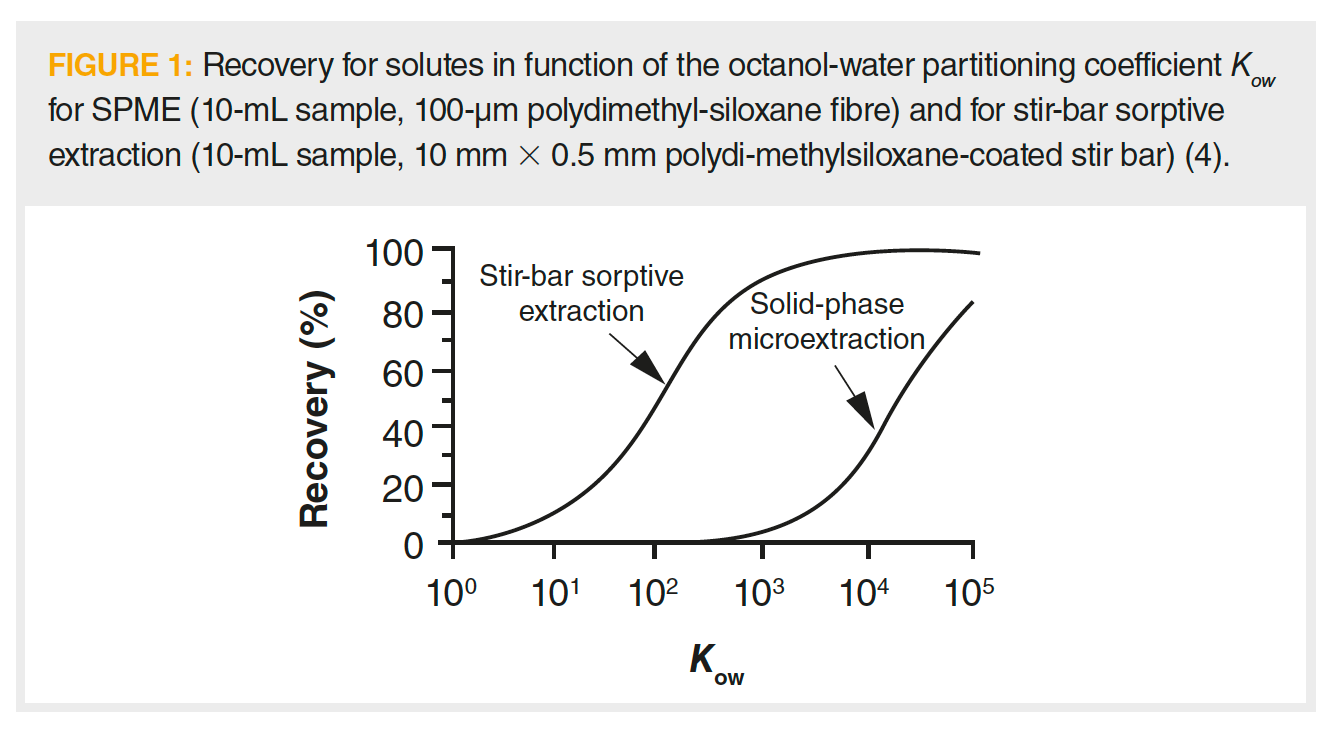
The stir bars used for SBSE have a much larger surface area than the fibres used in SPME. Consequently, they contain on the order of 50–250 times more extracting phase (sorbent). With such an advantage in terms of phase ratio, SBSE is expected to separate various classes of solute with lower Kow and greater amounts of solutes with similar Kow.
However, this advantage of greater amount of solute extraction comes at a price. Both the sorption and desorption steps in SBSE are significantly slower than in SPME, though automation may help address the sorption and desorption steps. Although other practitioners may disagree, I contend that SPME is preferred when gas chromatography (GC) is the subsequent analysis step, and SBSE is preferred when liquid chromatography (LC) is used (though either approach can be used for any type of chromatography). However, as a result of this bias, it is important to remember that, with biological samples, macromolecules such as sugars and proteins may need to be washed from the sorbent phase in SBSE, while this is less likely the case in SPME. Both SPME and SBSE are non-exhaustive equilibrium-based extractions, meaning that calibrations must be determined during the method development phase to determine the extraction duration to achieve equilibrium. Finally, it should be noted that newer configurations of SPME, such as the SPME-Arrow, have increased sorbent amounts, though still less than found in SBSE.
Question 6: Consider an ionic compound, MX, composed of the cation M+ and the anion X-, which dissociates in water. Write the equilibrium equation for each equilibria in an extraction system.
Consider an organic extracting phase (org) and an aqueous sample phase (aq). The equilibria between each species in each phase must be considered:
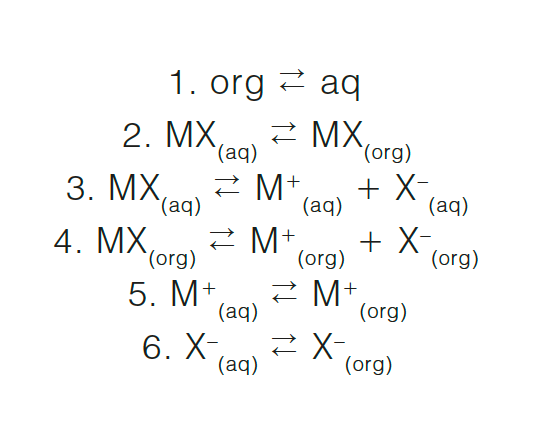
Depending on the dissociation constant of MX(aq) and the sizes of the relevant equilibria constants, it is likely that only equilibria 2 and 3, and perhaps 1, will be important in an analytical extraction. For some polyatomic systems such as the bicarbonate-carbonate system, all dissociations (H2CO3 HCO3- CO32-) must be considered.
Conclusion
I hope these exam questions over the past couple of columns have been fun and educational. I enjoyed putting them together. Now, with our next instalment, we’ll return to more focused treatments of specific sample preparation techniques and applications.
References
- Schul, D.; Tallmadge, D.; Burress, D.; et al. Determination of Fat in Olestra-Containing Savory Snack Products by Capillary Gas Chromatography. J. AOAC Intl. 1998, 81 (4), 848–868.
- US EPA Method 625, Revision A, Federal Register 80 (33), 8956 (19 February 2015).
- Ebitson, M.; Gallagher, D.; Cannon, A.; et al. New Method US EPA 625 with Solid Phase Extraction for Challenging Wastewaters. LCGC North Am. Supplement 2015, 33 (12s), 66.
- Sandra, P.; David, F.; Tienpont, B. Stir‑Bar Sorptive Extraction of Trace Organic Compounds from Aqueous Matrices. LCGC North Am. 2003, 21 (2), 108–118.
Douglas E. Raynie is a department head and associate professor at South Dakota State University. His research interests include green chemistry, alternative solvents, sample preparation, high‑resolution chromatography, and bioprocessing in supercritical fluids. He earned his Ph.D. in 1990 at Brigham Young University under the direction of Milton L. Lee. Raynie is a member of LCGC’s editorial advisory board. Direct correspondence about this column via e-mail to amatheson@mjhlifesciences.com

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)





