The Chromatographic Society Meeting Report: Supercritical and Ultrahigh-Pressure Chromatography: Part 2
The three-day Chromatographic Society meeting was held on Monday 15th until Wednesday 17th May 2017 and was hosted by Pfizer at Discovery Park in Sandwich, Kent, UK. This comprehensive symposium featured oral presentations from leading practitioners of supercritical fluid chromatography (SFC) and ultrahigh-pressure liquid chromatography (UHPLC) from throughout Europe. Some of the latest innovations and applications were described and the lectures were augmented and supported by a comprehensive exhibition of instrumentation and consumables. The attendees gained insight into practical application of these techniques across a variety of industries-particularly the pharmaceutical industry. As was anticipated, in a comprehensive programme there was a significant focus on SFC for chiral and preparative-scale analysis.
Photo Credit: plule_r/Shutterstock.com

The three-day Chromatographic Society meeting was held on Monday 15th until Wednesday 17th May 2017 and was hosted by Pfizer at Discovery Park in Sandwich, Kent, UK. This comprehensive symposium featured oral presentations from leading practitioners of supercritical fluid chromatography (SFC) and ultrahigh-pressure liquid chromatography (UHPLC) from throughout Europe. Some of the latest innovations and applications were described and the lectures were augmented and supported by a comprehensive exhibition of instrumentation and consumables. The attendees gained insight into practical application of these techniques across a variety of industries-particularly the pharmaceutical industry. As was anticipated, in a comprehensive programme there was a significant focus on SFC for chiral and preparative-scale analysis.
After an evening social activity at the historic Dover Castle (Figure 1), the Wednesday morning session was chaired by John Lough (University of Sunderland). He also provided the first presentation of the day, introducing himself and his presentation: “UHPLC in Practice: Some Vignettes From TeachingâLed Research”. He discussed the various applications of the ultrahigh-pressure liquid chromatography (UHPLC) instrumentation in his teaching laboratory at the University including pharmaceutical method development (1), which allowed a 60-min European Pharmacopoeia (Ph. Eur.) method for paroxetine-related substances to be analyzed in 2 min (it required a ternary mobile phase incorporating 5% [v/v] THF). Other areas of research included method development for medicinal creams and lotions and recreational drugs of abuse.

John also discussed the possibility of obtaining selectivity changes through any changes of k with increasing pressure as previously reported by McCalley (2). In general, no changes in selectivity were observed for a number of compound classes as pressure was increased (whether through use of capillary restrictors of varying lengths, increased flow rate, or coupling columns). However, John did present an example of the antihistamine, chlorpheniramine, and an impurity at a mobile phase pH close to its pKa value where increasing the flow rate improved resolution. He also advocated the use of temperature, in some cases up to 95 °C, to speed up separations, but had only found one instance where this had been accompanied by a significant change in selectivity.
He completed his lecture by discussing measurement of extra-column band broadening and noted that the efficiency benefits of subâ2âµm core–shell particles could be obtained even on early UHPLC systems with moderate system dispersion. However, this was only possible at relatively high k values, which could be considered impractical. He also advocated the use of isocratic initial holds on chromatographic methods to stack and focus samples, which was particularly effective for highly aqueous sample diluents.
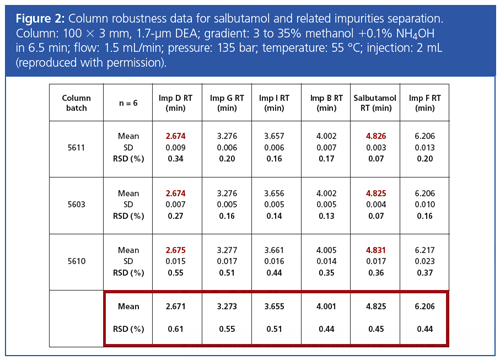
In a switch back to supercritical fluid chromatography (SFC), Amandine Dispas (Liege University) spoke eloquently on “Implementation of SFC for the Quality Control of Pharmaceuticals”. She initially discussed ICH guidelines pertaining to method development (Q8) including the use of “design spaces” (condition space under which a chromatographic method is robust) and method validation (Q2). Her research investigated whether it was possible to implement these guidelines in development and validation of an SFC method for antibiotics (3). This was investigated using an experimental design for the resolution of antimalarial compounds (4), which showed good peaks shapes with a diethylamine (DEA) stationary phase. She also discussed the analysis of salbutamol drug substance (5), which was analyzed under SFC conditions in a seventh of the time of the current Ph. Eur. liquid chromatography (LC) method.
The Ph. Eur. method for the analysis of this substance is an ion-pairing method using a phosphate buffer and sodium heptane sulfonate. Following column screening, this method was redeveloped using SFC. Retention time reproducibility was found to be good (%RSD <0.6) and an experimental design showed the method to be robust (see Figure 2 for column robustness data).
Similarly, a Ph. Eur. normal phase method for the analysis of cholecalciferol was redeveloped using SFC (6). Amandine then went on to describe the development of an SFC–mass spectrometry (MS) method for the analysis of an oil suspension of vitamin D. The experimental design included MS factors such as make-up flow composition and capillary and cone voltages.
She concluded the presentation by highlighting the increasing number of publications using SFC for quality control analysis, which has shown an increasing annual trend since 2013.
The next presentation of the morning was from Robbie Mutton (University of Cambridge), who discussed “Supercritical Fluid Flow Chemistry and At Line Analysis”. He introduced the work conducted in Stephen Ley’s group on flow chemistry by discussing the basics of this synthetic chemistry approach (7) and the development of the flow chemistry hardware. Reproducible flow through the four columns was achieved through the use of a restrictor after the carbon dioxide cylinder to maintain a constant inlet pressure. He exemplified this system through the use of this system on a Claisen rearrangement (8), which can process 45 g of material every 24 h. He completed his presentation by describing the interfacing of MS detection to further increase the utility of this system and how this whole system can be controlled via an in-house developed Android app allowing remote control and troubleshooting of the system.
In one of a number of presentations describing preparative SFC in support of discovery chemistry, Maria Luz de la Puente (Eli-Lilly) presented her group’s work on “Open Access Chiral Chromatography: Isomers À La Carte”. The analytical system described used 0.2% (v/v) isopropylamine (good UV transparency), but the semiâpreparative system used 0.2% (v/v) dimethylethylamine (good volatility for postâisolation sample work-up). She then went on to exemplify this system and introduced the simplified open access software interface allowing facile use of the technology. The software can automatically calculate isocratic semi-prep conditions based on the analytical chromatographic conditions to further streamline the process.
In a similar presentation, Stephan Buehler discussed “Separation Support for Medicinal Chemistry at Boehringer Ingelheim”. Stephan’s laboratory has a service level agreement of three days from receipt of sample to purified compound. The samples require presentation of 50 mg concentration of the desired analyte in a maximum of 5 mL solvent and are registered through an automated bar code system into a purification management system (“PUMA”). The use of a pre-screening LC–MS platform allows the automated prediction of a semi-prep gradient profile for compound isolation. Nitrogen evaporation or freeze drying are used for solvent removal. This process was used on 5820 samples during 2016. To end the presentation, he briefly noted the implementation of SFC–MS to further improve this workflow.
In a change of focus Frederic Lynen (University of Ghent) presented his group’s research on “Possibilities and Limitations of Mixed and of Hydrothermally Stable Stationary Phases Allowing Alternative Solutions in Supercritical Fluid Chromatography”. Frederic opened his lecture by noting that supercritical fluids are compressible and therefore density is a critical parameter in analyte elution in SFC. He discussed his group’s work on utilizing kinetic plots to describe the separation boundaries of SFC. However, as variation of flow rate changes system pressure, adjustment of the back pressure regulator was necessary to maintain mobile phase density for these calculations. From these numbers he noted (a) the need for a 1200 bar SFC system to utilize sub-1-µm particles, (b) for high speed separations SFC should be preferred over LC, and (c) for high efficiency separations LC should be utilized.
Frederic then went on to describe his group’s work on stationary phase optimized selectivity LC (SOSLC) as previously described by Nyiredy (9). For this to be implemented in SFC, reliable retention factors are required for modelling and these are generated under isopycnic conditions. He illustrated this for the chiral separation of transâstilbene oxide.
He then moved onto SFC using pure water as mobile phase and synthetic diamonds (from the mining industry) as stationary phase. The synthetic diamonds are able to tolerate the extreme temperatures and pressures required to make water supercritical (produced using a gas chromatography [GC] oven). He noted that the surface of synthetic diamonds is very polar because no retention was observed under reversed phase conditions-slightly unexpected from a purely carbon-based stationary phase! He exemplified the use of supercritical water for the separation of polyaromatic hydrocarbons (370 °C and 400 bar pressure) but retention was low. This approach could only be used for extremely stable molecules!
The afternoon sessions were opened by Hania Khouri (Agilent) discussing “Application of Supercritical Fluid Chromatography (SFC) coupled to Ion Mobility Mass Spectrometry (IM-MS) in the Analysis of Lipids”.
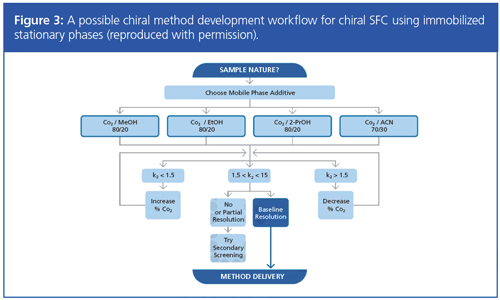
In a return to chiral SFC, Pilar Franco (Chiral Technologies Europe) gave a presentation titled “Daicel and Chiral SFC (Experience from an Indication of Continuous Use)”. An example of one of the workflows presented utilizing immobilized stationary phases is shown in Figure 3.
Regular presenter at Chromatographic Society meetings, Chris Titman (Shimadzu) continued one of the key themes of the meeting: “Perfect Partners, Why SFC–MS Matters to Your LC–MS Analysis”. Chris started his presentation by providing an overview of the chiral insecticides used in this work and then illustrated why reversedâphase LC–MS is the current technique of choice for analyzing these types of molecules. He then went on to show that SFC–MS is much more sensitive than LC–MS for these same general analytes-but posed the question “Does SFC provide a generic solution for the analysis of insecticides?”
As with LC–MS, he illustrated that low buffer concentration (ammonium formate) and a methanol make-up flow at low flow rate (0.1 mL/min) was preferable for best sensitivity; the response was shown to be largely independent of the concentration of organic solvent in the bulk mobile phase. In terms of capillary (interface) voltage a high (5 kV) value for SFC–MS was required compared to LC–MS (to produce the approximate same signal intensity), which was thought to be because of the additional energy required to form protonated species in the absence of water. Adding between 5% and 50% (v/v) water to the make-up flow eluent gave very similar SFC–MS responses at 2 and 5 kV capillary voltages and appeared to add little benefit. Interestingly, temperatures for the transfer line and MS interface were of similar magnitude and effect on signal response for both LC–MS and SFC–MS.
In terms of sensitivity and applicability, Chris went on to discuss why the improved sensitivity from SFC was useful in terms of recovery in complex vegetable matrices, but some analytes were better suited to LC than SFC analysis and vice versa with regards to recovery.
Chris then moved on to SFC as an alternative approach to hydrophilic interaction liquid chromatography (HILIC) for analyzing polar insecticides and biosides-illustrating it was a very viable approach.
The vendor presentations continued through Jane Cooper (Waters) discussing the “Analysis of Cosmetic Allergens in Perfumes, Cosmetics, and Personal Care Products using Ultra Performance Convergence Chromatography (UPC2) with MS Detection”. Jane opened her presentation by discussing the uses and applications of fragrances and the allergic reactions these can lead to in certain instances. She then went on to briefly discuss EU regulations and current methods for
analysis, which are dominated by GC–MS approaches.
Cosmetic allergens can cover a range of structures and polarities suited to SFC (30 were presented in this work). In addition, the use of SFC can mitigate the use of many hazardous solvents such as DCM often used in the equivalent GC analyses. Jane then went on to discuss the hardware (a triple quadrupole MS) and the method development screening process used. This involved column selection followed by organic modifier type. Detection limits were found to be better with atmospheric pressure chemical ionization (APCI) compared to electrospray. She concluded her presentation by highlighting a number of separations with low level detection afforded by the use of MRM transitions on the MS.
In a change of direction, Lynne Hunter (Pfizer) discussed her company’s efforts to automate their UHPLC method development process in her presentation “Autochrom and Beyond: Software-Assisted UHPLC Method Development”. Pfizer’s method development process is developed through a series of experiments termed waves. The initial wave (0) principally involves accumulating pertinent analyte physicochemical data and samples. The “key predictive sample set” (KPSS) includes actual and theoretical impurities, degradants formed through purposeful degradation experiments, and degradants formed under accelerated stability conditions (higher temperature and humidity than standard purposeful degradation studies) and may include both stressed drug substance and drug formulations.
Once the KPSS is established in wave 1, an automated screening approach is used. This involves the screening of four column types at three pHs (2.7–0.1% [v/v] formic acid, 6.8–10 mM ammonium acetate, and 10.7–0.1% [v/v] ammonium hydroxide) with methanol and acetonitrile as organic solvent. At this point the pH is optimized using the data from the three different mobile phases screened, but the mobile phase is maintained to allow peak tracking via MS.
In wave 2, a standard temperature versus gradient time approach is taken and the data modelled in-silico. In the final wave, fineâtuning of the mobile phase pH is undertaken (± 1 pH unit) to optimize selectivity and the point of optimal method robustness.
The optimized methods can also be used for the predictive modelling of new or theoretically possible impurities and degradants with reasonable retention time prediction.
Returning to vendor presentations, Timothy Cross (Thermo Fisher Scientific) discussed his company’s principal UHPLC system: “A New Paradigm in Separation Performance: The Thermo Scientific Vanquish UHPLC Platform”.
The final two presentations of the afternoon revisited SFC in pharmaceutical discovery environments. The first was provided by Jenny Kingston (AstraZeneca) on the “Flexible Applications Using Supercritical Fluid Chromatography Coupled to Triple Quadrupole MS to Support of Drug Discovery Projects”. Jenny started her presentation by outlining the various UHPLC and SFC analytical and preparative systems (and sample postâprocessing) instrumentation for the analysis of both chiral and achiral molecules.
Rapid method (<2 min analysis time) openâaccess screening systems based on SFC and HPLC with multiple columns are available to synthetic chemists. Impressive orthogonality was demonstrated for the UHPLC method (a positively surface charged organo-silane hybrid C18) versus an SFC method using a 2-ethylpyridine phase when the positively surface charged organoâsilane hybrid C18 column was run at low pH and the 2-ethylpyridine column at high pH (retention correlation coefficient [R2] = 0.02). The orthogonality of columns with SFC was also demonstrated as part of a method development toolkit. Again, with a 2-ethylpyridine and a HILIC diol column, a retention orthogonality value of 0.16 was possible with the same mobile phase and illustrated the diversity of stationary phase selectivity in SFC.
Jenny then went on to exemplify the power of SFC by comparing the separation of a compound and its isomeric impurities which exhibited coelution. When transferred to SFC, these were easily resolved and scalable to semi-preparative conditions. She also presented an example where water-labile compounds were analyzed successfully with SFC without degradation. Other examples of the application of SFC–MS and SFC–MS/MS were also shown including separation of polar nucleosides and nucleobases, chiral in vivo analysis to support DMPK studies, low level impurity quantification (MS/MS detection), atropisomer separation, and cell samples for quantitative stability measurements of included drugs.
She concluded her talk with discussion of the lab-2-lab sample transport and analysis system, which will be installed in AZ’s new Cambridge Biomedical Centre campus. This will allow the transport of samples from the laboratory to a central testing area in an automated fashion.
The final presentation of the symposium was provided by Alex Bozic of Absys SFC describing “A New Era in Preparative SFC”. Alex discussed his work in providing a mechanism to convert “nearly every” prep LC instrument into a prep SFC system controllable through the native LC software. He started his presentation by discussing some of the issues with SFC sample collection such as poorer recoveries and increased likelihood of sample crossâcontamination compared to normalâphase LC approaches. He then went on to describe the issues with performing high throughput prep SFC and noted only one current commercial system could do this.
Alex described how this project had started in 2012 at Sanofi-Aventis and five years later this prep LC-now prep SFC system runs 10,000 compounds a year. He noted that both prep HPLC and SFC uses the same hardware, the same control software, share the same structural database repository, and have the same workflow. He also noted that the modification of the prep LC to produce the prep SFC was only possible because of a patented high performance gas-liquid separator and additional modules like heat exchangers, pump head cooling device, and a back pressure regulator, which were independently controlled by a PC.
Upon conclusion of the meeting Chris Bevan (The Chromatographic Society’s Events Coordinator and the lead organizer for this meeting) drew the meeting to a close by thanking the hosts Pfizer, the vendor companies who had sponsored the event, and the colleagues who attended. The meeting provided a comprehensive and informative snapshot of the application of SFC and UHPLC and research in these areas and all attendees will have left knowing significantly more about these fields.
References
- R.W.H. Perera and W.J. Lough, J. Liq. Chromatogr.36, 349 (2013).
- D. McCalley et al., J. Chromatogr. A1217(3), 276–284 (2010).
- A. Dispas et al., J. Chromatogr. A1353, 78 (2014).
- A. Dispas et al., J. Pharm. Biomed. Anal.125, 339 (2016).
- A. Dispas et al., J. Pharm. Biomed. Anal.134, 170 (2017).
- B. Andri et al., J. Chromatogr. A1491, 171–181 (2017).
- S. Newton et al., Angew. Chem. Int. Edn.53, 4915 (2014).
- T. Ouchi et al., ACS Sustainable Chem. Eng.4, 1912 (2016).
- S. Nyiredy et al., J. Chromatogr. A.1157, 122 (2007).
E-mail:chromsoc@meetingmakers.co.ukWebsite: www.chromsoc.com

New Study Investigates Optimizing Extra-Column Band Broadening in Micro-flow Capillary LC
March 12th 2025Shimadzu Corporation and Vrije Universiteit Brussel researchers recently investigated how extra-column band broadening (ECBB) can be optimized in micro-flow capillary liquid chromatography.
Separating Impurities from Oligonucleotides Using Supercritical Fluid Chromatography
February 21st 2025Supercritical fluid chromatography (SFC) has been optimized for the analysis of 5-, 10-, 15-, and 18-mer oligonucleotides (ONs) and evaluated for its effectiveness in separating impurities from ONs.








