Characterization of Adeno-Associated Virus (AAV) Gene Therapy Products
Enumerable types of gene therapies are collectively one of the fastest-growing areas of biopharmaceutical products today. Gene therapies are generally defined as treatments, perhaps even cures, for diseases through the transfer of genetic material to host cells. The analytical technologies that exist to characterize these novel therapies are rapidly advancing. In this column, we will briefly present some of the basic concepts related to this novel product class, specifically related to formulation and delivery, focusing on adeno-associated virus (AAV) formulations. We will then briefly discuss the analytical technologies and approaches most commonly used to characterize these products, specifically on the characterization of AAV-related products. We will briefly discuss what many consider the gold standard in analytical tools, analytical ultracentrifugation (AUC), and then specifically discuss liquid chromatography-mass spectrometry (LC–MS) tools and methods (such as the multi-attribute method, MAM) that are becoming more commonly used in characterization.
Gene therapy is a procedure that enables gene improvement by means of the correction of the altered (mutated) genes, or targets site-specific modifications that have therapeutic treatment as a target molecule (1). The most common vectors are viruses that have evolved a way of enveloping and delivering their genes to human cells. The most widely used gene transfer vectors in clinical trials are adenoviral vectors, retroviral vectors, adeno-associated viruses, and herpes simplex viruses (1). Around 800 approved gene therapy clinical trials worldwide have been conducted or are still ongoing; a large majority of these trials use a viral vector for gene transfer (drug delivery) (2).
Adeno-associated viruses (AAV) belong to the parovirus family, and AAV has emerged as one of the main vectors used in gene therapy due to many useful attributes, including a lack of pathogenicity and continuous maintenance of the viral genome (3). The AAV viral vector consists of a protein shell protecting a small, single-stranded DNA genome of approximately 4.7 kilobases (kb). Efficient replication and viral gene expression of the AAV genome requires co-infection with a second helper virus, such as adenovirus or herpes. The single-stranded genome contains three genes, Rep (Replication), Cap (capsid), and Aap (assembly). The coding sequences are flanked by inverted terminal repeats (ITRs) that are required for genome replication and packaging. The AAV capsid consists of 60 copies of three types of viral proteins (VPs), VP1, VP2, and VP3, assembled in a ratio of 1:1:10 (VP1/VP2/VP3). The three VPs are encoded by the cap gene, alternatively spliced from one mRNA. There are over 100 naturally occurring AAVs that have been isolated in humans and animals, which differentiate from each other based on their capsid components, cellular tropism, transduction efficiency, and immunogenicity. Within the same viral serotype, the VPs share a common protein sequence (3–6).
AAV delivery technologies have led to the development of two FDA-approved gene therapy products. Luxturna, an AAV2-based gene therapy for a rare, genetic form of blindness, was approved in 2017 (7), and Zolgensma, an AAV2-based gene therapy to treat spinal muscular atrophy (SMA), was approved in 2019 (8).
AAV Viral Vector Manufacturing
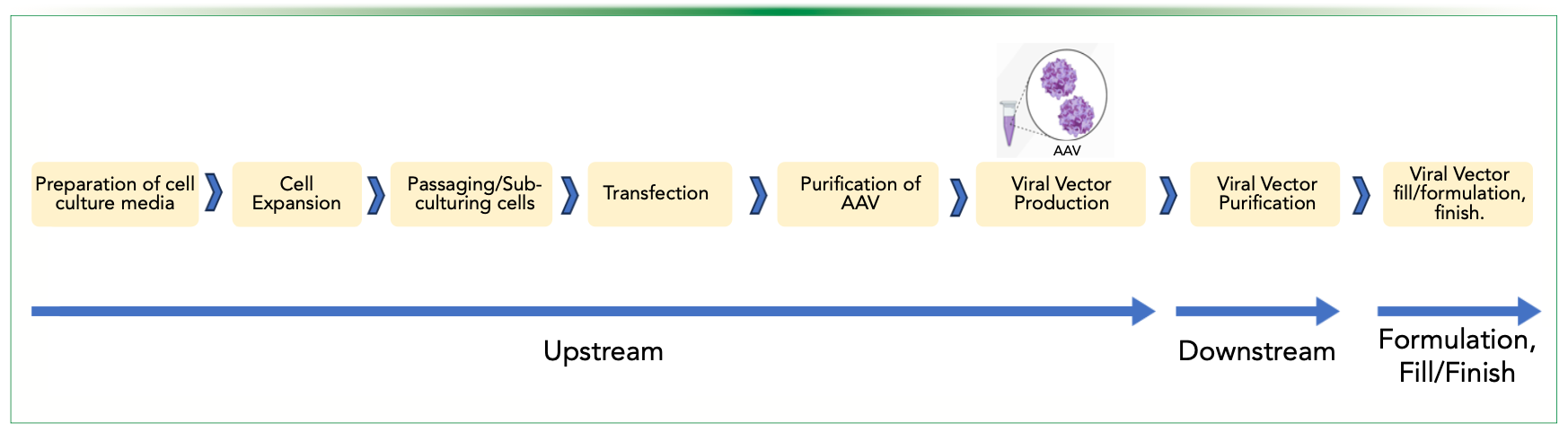
Development of scalable and robust manufacturing processes is necessary for gene therapy products that meet clinical and market demands and achieve cost of goods targets (4,5). AAV viral vector manufacturing is a complex process that necessitates new approaches to meet the challenges of stability, safety, efficacy, quality, preventing degradation of AAV vectors during manufacturing, handling, and storage as well as compiling with the current Good Manufacturing Practice (cGMP) (4,5). Viral vector manufacturing workflows include upstream, downstream, formulation, and fill or finish processing unit operations as shown in Figure 1.
FIGURE 1: Viral vector manufacturing process workflow, which involves various upstream, downstream, formulation, and fill/finish steps. Figure is adapted from reference (4).
Upstream Processing
Unit operations in upstream viral vector production include: (i) plasmid development and production that requires a cis-plasmid, a trans-plasmid (that encodes the AAV rep and cap genes), and a helper plasmid (that encodes adenovirus [Ad] helper genes, E2A, E4, and VA RNA); (ii) cell expansion where E1 transduced cells (such as adherent HEK293T [a human embryonic kidney cell line that is typically used for AAV manufacturing] or suspension HEK293) are grown to a desired cell density; (iii) plasmid transfection where plasmids are introduced into cells; and (iv) viral vector production where transiently transfected cells produce the virus. While transient plasmid DNA transfection is adaptable to new AAVs and produces high particle yields, large amounts of plasmid DNA are needed to scale up for larger vector batches (4,9,10).
Downstream Processing
The downstream process includes methods for purification of viral particles from process- and product-related impurities, including host cell material, plasmid DNA, and empty capsids. A typical downstream process includes: (i) cell lysis; (ii) removal of nucleic acid; (iii) centrifugation or microfiltration to remove cell fragments and debris; (iv) affinity chromatography; (v) separation of full gene-containing infectious viruses from empty, non-infectious viruses, using cesium chloride gradient ultracentrifugation procedures or ion-exchange chromatography (IEC), and (vi) further purification by reduction of host cell proteins using core-bead adsorbents (4,9,10).
Methods for Downstream Processing
The AAV product manufacturing process can introduce many impurities throughout the manufacturing process such as (but not limited to) non-encapsulated DNA, empty capsids, aggregated capsid, and residual proteins, and some of these are concerns for immunotoxicity (11).
The major challenge of the AAV manufacturing process is production of capsids that are not packaged with the therapeutic transgene and are therefore referred to as empty capsids. The empty capsids are considered as a product-related impurity, and could potentially cause impairment of potency through receptor competition. Along with empty capsids, partially filled capsids are also produced during the manufacturing process and may contain packaged process-related impurities or truncated genomes. This necessitates the use of analytical techniques that can provide information regarding the content distribution of AAV capsid content (12).
Several studies have evaluated analytical methods for their ability to assess capsid content, as the measurement of AAV product is extremely challenging. These methods include density gradient separation using analytical ultracentifugation (AUC), cryo-electron microscopy (cryo-EM), liquid chromatography-mass spectrometry (LC–MS), size exclusion chromatography coupled to multiangle light scattering (SEC-MALS), capsid titre-to-genome titre ratio using q-PCR, and anion-exchange chromatography (AEX) (4,12–14).
Analytical Ultracentrifuge (AUC)
Analytical ultracentrifuge (AUC) is a high-resolution technique and has become the gold standard for AAV quantification (15–17). It can be used to analyze capsid content through the difference in buoyant density due to differences in mass (15–17) between empty and full capsids.
AUC measures the concentration gradients of sedimenting molecules during the application of a centrifugal force while maintaining temperature and rotational speed. It is equipped with the interferometry optical system that allows the determination of concentration gradients for macromolecules in solution. Full capsids have a sedimentation coefficient c(s) of approximately 105 s, whereas empty capsids have values of approximately 66 s. As full capsids have a greater buoyant density than empty capsids, they sediment more quickly through solution (18).
A single sedimentation velocity run on an adenovirus sample can be used to detect and quantify different forms of virus particles and subviral particles. These forms include (i) intact virus monomer particles; (ii) virus aggregates; (iii) empty capsids (ECs); and (iv) smaller assembly intermediates or subparticles formed during normal or aberrant virus assembly. The data can be obtained by direct boundary modeling of the AUC data generated from refractometric or UV detection systems to provide characterization details (18–20). However, the major challenge for the AUC method is the requirement of large quantities of material along with a low throughput.
Mass Spectrometry (MS)
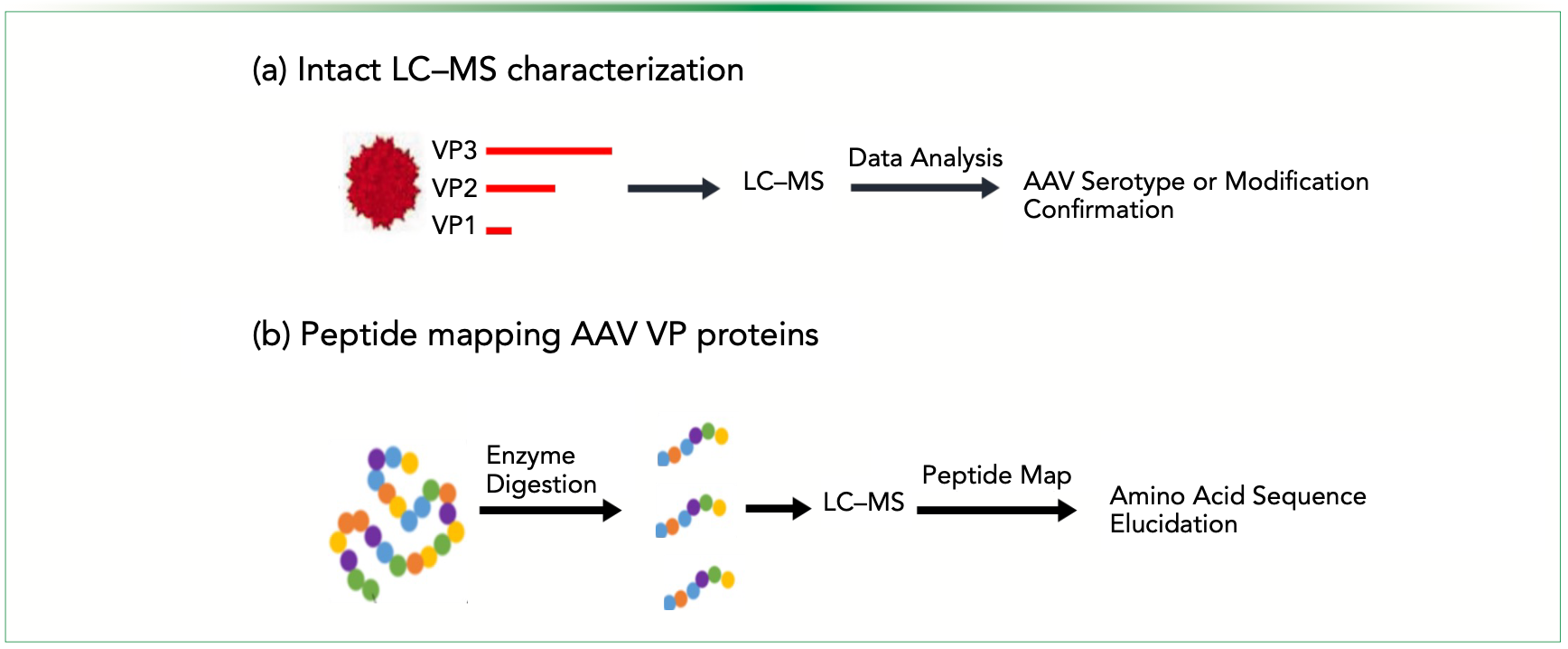
Mass spectrometry (MS) has emerged as a high-resolution analytical technique in protein characterization. LC–MS offers a robust method to directly measure the protein molecular weight of the AAV capsid, providing both specificity and sensitivity for rapid confirmation of capsid serotypes. Peptide mapping methods for different AAV capsids (including chemically engineered capsids) have been optimized for high sequence coverage and precise amino acid structure identification using tandem mass spectrometry (MS/ MS), and are being used for capsid protein characterization and process impurity identification (21), as shown in Figure 2.
FIGURE 2: Schematic diagrams of AAV capsid characterization workflow: (a) Intact LC–MS analysis, and (b) and peptide mapping amino acid sequence of VP proteins. Figure adapted from reference (21).
Intact AAV Capsid Characterization Using LC–MS
A unique application of MS is the intact AAV analysis and its ability to differentiate fully filled (capsids containing the genes of interest) versus partially filled or empty capsid particles also referred to as the content ratio. The capsid of each AAV serotype consists of 3 VP proteins: VP1 (82kDa), VP2 (66kDa), and VP3 (60kDa), which share an overlapping open reading frame. Analytical LC–MS and LC-coupled tandem MS (LC–MS/MS) provide specificity, sensitivity, and speed in analysis in less than 5 min. Intact LC–MS can be used to determine the exact mass gain for capsid chemical modification, thus providing information of the AAV capsid (21,22).
Compared with other methods such as AUC, SEC, and IEC, native MS has the advantages of minute sample requirements, rapid analysis, high sensitivity, and high mass resolution to distinguish between full, partially full, and empty capsids. However, one of the main challenges in native MS analysis of AAV vectors is poor resolution of viral particles containing variable lengths of the genomic content (22).
Peptide Mapping Analysis of AAV Capsids using LC–MS/MS
Characterization of AAV viral proteins includes the evaluation of capsid stoichiometry, identity, and post-translational modifications (PTMs). These parameters of the viral capsid are the critical quality attributes that can influence the tropism, infectivity, and transduction efficiency of the AAVs (21). LC–MS/MS is one of the most valuable and advanced tools in analytical chemistry to conduct a comprehensive peptide map analysis including post-translational modifications as it is high-throughput, amenable to quality control, has great specificity, and speed to ensure the quality and safety (contaminants in each AAV batch will be detected in LC) of the final vector product. Coupling MS/MS further allows to peptide map the precise amino acid sequence of each capsid protein, including chemical conjugations, and the chemistry of the AAV capsid proteins. Peptide mapping analysis of the AAV capsid proteins has been shown to achieve high sequence coverage and precise amino acid structure elucidation, including the identification of covalent modifications (22).
Multi-Attribute Characterization
While the separation of VP3 is achieved, distinguishing VP1 and VP2 is challenging as they share a common C-terminal sequence. Integrating a multi-attribute characterization of AAVs using a two-dimensional liquid chromatography (2D- LC) coupled with MS can be used to perform comprehensive and high-throughput analysis. This approach allows for different chromatography techniques to be coupled with MS for analytical characterization of biomolecules (23). The multi-attribute method (MAM) is a LC–MS-based method that is used to characterize and monitor product quality attributes (PQAs) at the amino acid level of a biopharmaceutical product. MAM relies on proteolytic digestion of the sample followed by reversed-phase chromatographic separation and high-resolution LC–MS analysis in two phases (24). There are two aspects of the MAM workflow—first, a discovery study to determine quality attributes for monitoring. This involves the creation of a targeted library based on high-resolution retention time along with an accurate mass analysis. The second aspect of MAM is the monitoring phase that relies on the target peptide library and new peak detection. It uses differential analysis of the data to determine the presence, absence, or change of any species that may affect the stability of the biotherapeutic.
The protocol consists of three main stages:
- Sample digestion, or proteolytic digestion of the protein.
- Reversed-phase chromatographic separation of the generated peptides and detection. During Phase I of MAM (discovery phase), data-dependent acquisition (DDA) MS/MS is performed to enable confident identification of peaks and development of a peptide library. During Phase II of MAM (monitoring phase), full MS acquisition is carried out for the monitoring of predefined PQAs or CQAs.
- Data processing, analysis, and reporting for each of the two phases.
Recently, a 2D-LC–MS platform that combined anion-exchange chromatography (AEX) and RPLC–MS was used to measure the amounts of empty and full capsids, characterize viral proteins, and evaluate capsid identity and PTMs. High-resolution separation in the first dimension was achieved using AEX, with a salt gradient to achieve empty and full capsid separation. Using the heart-cutting technique in 2D-LC, peaks of interest were then transferred and stored in different trapping loops. The fractions were sequentially transferred to second-dimension LC, where they were subjected to online denaturation and desalting in a trap column. The AAV capsid was denatured by online acidification, and the MS-incompatible salt was then removed. VPs were separated by RPLC in the second dimension, followed by MS analysis (23).
The main advantage of the method is the high-throughput and multi-attribute characterization of the sample in a single experiment. The method demonstrates the ability to perform online fractionation and subsequent downstream analysis in an integrated manner. Integration of additional methods will enable multi-attribute characterization in a single experiment with minimal sample handling required for different AAV serotypes (24). MAM can be applied throughout the product life cycle, from process development through upstream to downstream processes up to quality control release, and can be used to enforce current Good Manufacturing Practice regulations.
References
(1) Cavazzana-Calvo, M.; Thrasher, A.; Mavilio, F. The Future of Gene Therapy. Nature 2004, 427, 779–781. DOI: 10.1038/427779a
(2) Clinical trials.gov. https://clinicaltrials.gov/ct2/results?cond=&ter m=AAV&cntry=&state=&city=&dist=. (accessed 09-29-2023).
(3) Wang, D.; Tai, P. W. L.; Gao, G. Adeno-Associated Virus Vector as a Platform for Gene Therapy Delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. DOI: 10.1038/s41573-019-0012-9
(4) Srivastavaa, A.; Mallelab, K. M. G.; Deorkara, N.; Brophy, G. Manufacturing Challenges and Rational Formulation Development for AAV Viral Vectors. J. Pharm. Sci. 2021, 110, 2609−2624. DOI: 10.1016/j.xphs.2021.03.024
(5) Wright, J. Manufacturing and Characterizing AAV-Based Vectors for Use in Clinical Studies. Gene Ther. 2008, 15, 840–848. DOI: 10.1038/gt.2008.65
(6) Samulski, R.J.; Muzyczka, N. AAV-Mediated Gene Therapy for Research and Therapeutic Purposes. Annu. Rev. Virol. 2014, 1 (1), 427–451. DOI: 10.1146/annurev-virology-031413-085355
(7) Luxturna prescription information. 2018. https://www.fda. gov/vaccines-blood-biologics/cellular-gene-therapy-products/luxturna (accessed 09-29-2023).
(8) Zolgensma prescription information. 2020. https://www.fda. gov/vaccines-blood-biologics/zolgensma (accessed 09-29-2023).
(9) Ayuso, E.; Mingozzi, F.; Bosch, F. Production, Purification and Characterization of AAV. Curr. Gene Ther. 2010, 10, 423–436. DOI: 10.2174/156652310793797685
(10) Mevel, M.; Bouzelha, M.; Leray, A. et al. Chemical Modification of the Adeno-Associated Virus Capsid to Improve Gene Delivery. Chem. Sci. 2020, 11 (4), 1122–1131. DOI: 10.1039/c9sc04189c
(11) FDA (2021) Cellular, Tissue, and Gene Therapies Advisory Committee September 2–3, 2021 Meeting Briefing Document.
(12) Penaud-Budloo, M., François, A., Clément, N., Ayuso, E. Pharmacology of Recombinant Adeno-Associated Virus Production. Mol. Ther.-Methods Clin. Dev. 2018, 8, 166–180. DOI: https://doi.org/10.1016/j.omtm.2018.01.002
(13) Chiorini, J. A.: Kim F.; Yang L.; Kotin, R.M. Cloning and Characterization of Adeno-Associated Virus Type 5. J. Virol. 1999, 73 (2), 1309–1319. DOI: 10.1128/jvi.73.2.1309-1319.1999
(14) Andari, J. E.; Grimm, D. Production, Processing and Characterization of Synthetic AAV Gene Therapy Vectors. Biotechnol J. 2020, 16 (1), e2000025. DOI: 10.1002/biot.202000025
(15) Yarawsky, A. E.; Zai-Rose, V.; Cunningham, H. M. et al. AAV Analysis by Sedimentation Velocity Analytical Ultracentrifugation: Beyond Empty and Full Capsids. Eur. Biophys. J. 2023, 52, 353–366. DOI: 10.1007/s00249-023-01646-z
(16) Werle, A. K.; Powers, T. W.; Zobel, J. F. et al. Comparison of Analytical Techniques to Quantitate the Capsid Content of Adeno-Associated Viral Vectors. Mol. Ther. Methods Clin. Dev. 2021, 23, 254–262. DOI: 10.1016/j.omtm.2021.08.009
(17) Meng, H.; Sorrentino, M.; Woodcock, D. et al. Size Exclusion Chromatography with Dual Wavelength Detection as a Sensitive and Accurate Method for Determining the Empty and Full Capsids of Recombinant Adeno-Associated Viral Vectors. Hum. Gene Ther. 2022, 33, 202–212. DOI: 10.1089/hum.2021.123
(18) McIntosh, N. L.; Berguig, G. Y.; Karim, O. A. et al. Comprehensive Characterization and Quantification of Adeno Associated Vectors by Size Exclusion Chromatography and Multi Angle Light Scattering. Sci. Rep. 2021, 11, 3012. DOI: 10.1038/s41598-021-82599-1
(19) Burnham, B.; Nass, S.; Kong, E. et al. Analytical Ultracentrifugation as an Approach to Characterize Recombinant Adeno-Associated Viral Vectors. Hum. Gene Ther. Methods 2015, 26, 228–242. DOI: 10.1089/hgtb.2015.048
(20) Berkowitz, S. A.; Philo, J. S. Monitoring the Homogeneity of Adenovirus Preparations (a Gene Therapy Delivery System) Using Analytical Ultracentrifugation. Anal. Biochem. 2007, 362, 16–37. DOI: 10.1016/j.ab.2006.11.031
(21) Lam, A. K.; Zhang, J.; Frabutt, D., et al. Fast and High-Throughput LC–MS Characterization, and Peptide Mapping of Engineered AAV Capsids Using LC–MS/MS. Mol. Ther.–Methods Clin. Dev. 2022, 27, 185–194, DOI: 10.1016/j.omtm.2022.09.008
(22) Zhang, X.; Jin, X.; Liu, L.; Zhang, Z.; Koza, S.; Yu, Y.; Chen, W. Optimized Reversed-Phase Liquid Chromatography/Mass Spectrometry Methods for Intact Protein Analysis and Peptide Mapping of Adeno-Associated Virus Proteins. Hum. Gene Ther. 2021, 32, 1501–1511. DOI: 10.1089/hum.2021.046
(23) Wu, Z.; Wang, H.; Tustian, A.; Qiu, H.; Li, N. Development of a Two-Dimensional Liquid Chromatography-Mass Spectrometry Platform for Simultaneous Multi-Attribute Characterization of Adeno-Associated Viruses. Anal. Chem. 2022, 94, 3219–3226. DOI: 10.1021/acs.analchem.1c04873
(24) Millán-Martín, S.; Jakes, C.; Carillo, S. et al. Comprehensive Multi-Attribute Method Workflow for Biotherapeutic Characterization and Current Good Manufacturing Practices Testing. Nat. Protoc. 2023, 18, 1056–1089. DOI: 10.1038/s41596-022-00785-5
About the Author
Anantdeep Kaur is a Postdoctoral Research Associate at the Biopharmaceutical Analysis Training Laboratory for the Department of Chemistry and Chemical Biology at Northeastern University, in Boston, Massachusetts.

About the Column Editors
Jared Auclair is an Associate Dean of Professional Programs and Graduate Affairs at the College of Science at Northeastern University, in Boston, Massachusetts. He is also the Director of Biotechnology and Informatics, as well as the Director of the Biopharmaceutical Analysis
Training Laboratory.

Anurag S. Rathore is a professor in the Department of Chemical Engineering at the Indian Institute of Technology in Delhi, India.


Biopharmaceutical Characterization in the Age of Artificial Intelligence
May 13th 2025AI-powered tools are enhancing precision, efficiency, and decision-making in biopharmaceutical development. Recently, Jared Auclair and Anurag Rathore explored AI's evolving role in biopharmaceuticals in detail.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)