Challenges in the Analytical Characterization of VLPs Through HPLC-Based Methods
Characterization and quantification of virus-like particles (VLPs) through high performance liquid chromatography (HPLC)-based methods are challenging because of their large size, structural complexity, internal structural heterogeneity, and instability. Analytical techniques are essential to monitor morphology and internal structural heterogeneity at each process stage. Common analytical tools used in VLP characterization are microscopic techniques (such as transmission electron microscopy [TEM], atomic force-field microscopy [AFM], cryo-electron microscope [cryo-EM]), biochemical techniques (SDS-PAGE, western blotting), and light scattering techniques (such as dynamic light scattering [DLS], nanoparticle tracking analysis [NTA], and size-exclusion chromatography coupled with multi-angle light scattering [SEC-MALS]). However, these techniques are semi-quantitative and do not address morphology and internal heterogeneity. Therefore, HPLC-based techniques are sensitive, robust, and offer better resolution. The purity and titer of VLPs at any process stage can be monitored by reversed-phase chromatography and morphology, and stability-related issues can be monitored by the combination of HPLC and light scattering techniques like SEC-MALS. Challenges in HPLC-based methods are choosing columns with the right pore size and surface chemistry and effective sample preparations, as VLPs are very unstable and prone to fragmentation at process stages and the low titre of the VLPs. This article discusses the challenges and effective solutions for HPLC-based analytical characterization of VLPs.
Virus-like particles (VLPs) are nanoparticle-sized, multimeric, self-assembled protein complexes. They are non-infectious, as they lack the genetic material of parental viruses (1). VLPs trigger both cell-mediated and humoral-mediated immune responses, and are safer and more effective than attenuated or inactive vaccines, as there are fewer chances of reversion. Therefore, VLPs are emerging as potential vaccine candidates. Apart from vaccines, VLPs are also used as gene carriers in gene therapy (2). The size of VLPs ranges from 22–200 nm. VLPs can be expressed in different host systems, such as bacteria, yeast, insect cell lines, plant cell lines, and mammalian cell lines (3). Determining the identity, purity, and potency of VLPs is a crucial step towards regulatory approval, as they hold potential as vaccine candidates. Analytical methods play a critical role in ensuring quality control, monitoring stability during long-term storage, and thereby managing the critical quality attributes (CQA) in vaccine development.
Commonly Used Analytical Techniques for VLP Characterization
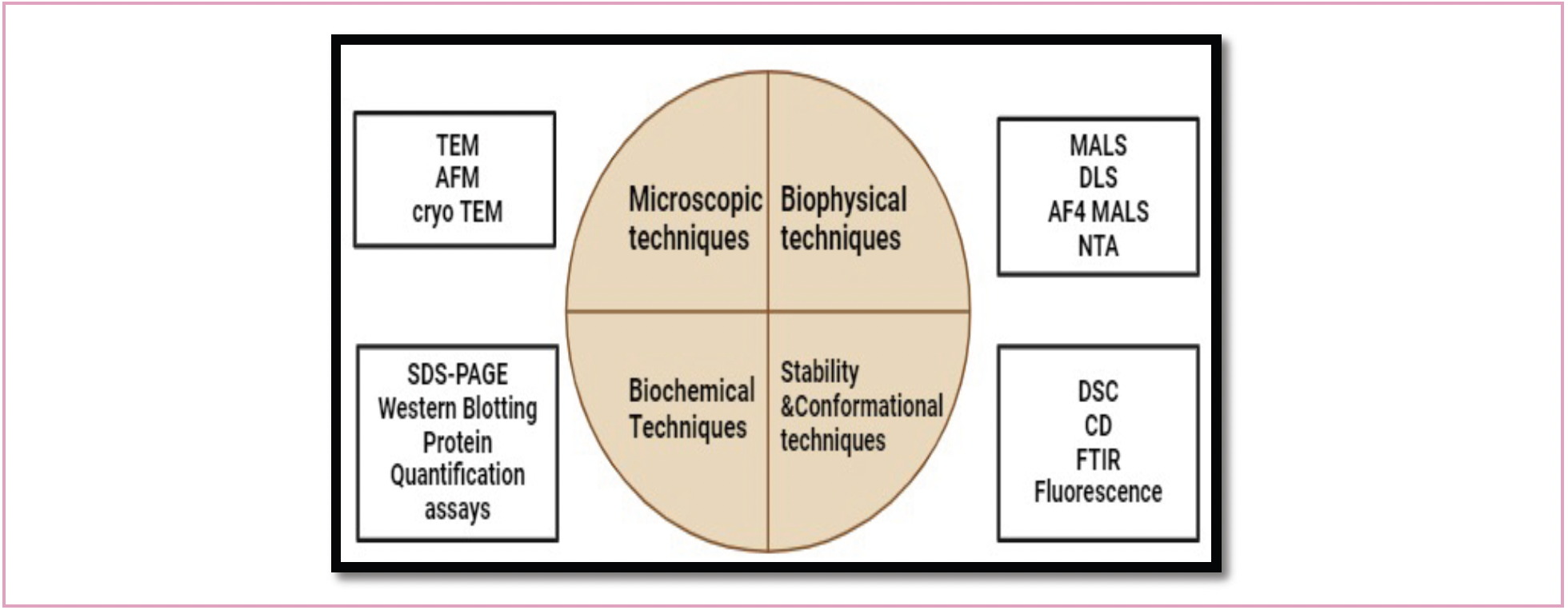
Microscopic techniques such as transmission electron microscopy (TEM), atomic force-field microscopy (AFM), cryo-electron microscope (cryo-EM), biophysical or light scattering techniques like dynamic light scattering (DLS), nanoparticle tracking analysis (NTA), size-exclusion chromatography coupled with multi-angle light scattering (SEC-MALS), asymmetric flow field fractionation coupled with multi-angle light scattering (AF4-MALS), and analytical ultracentrifugation (AUC) are commonly used to measure the morphology of VLPs (4). Biochemical techniques like sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting are commonly used for composition analysis of VLPs, and are quantified through densitometry. Other common techniques used for the quantification of VLPs are bicinchoninic acid assay, Bradford assay, and Lowry assay. Circular dichroism (CD) and differential scanning calorimetry (DSC) are also used to monitor thermal stability of VLPs (5) (Figure 1).
FIGURE 1: Analytical techniques for VLP characterization.
Challenges in VLP Characterization
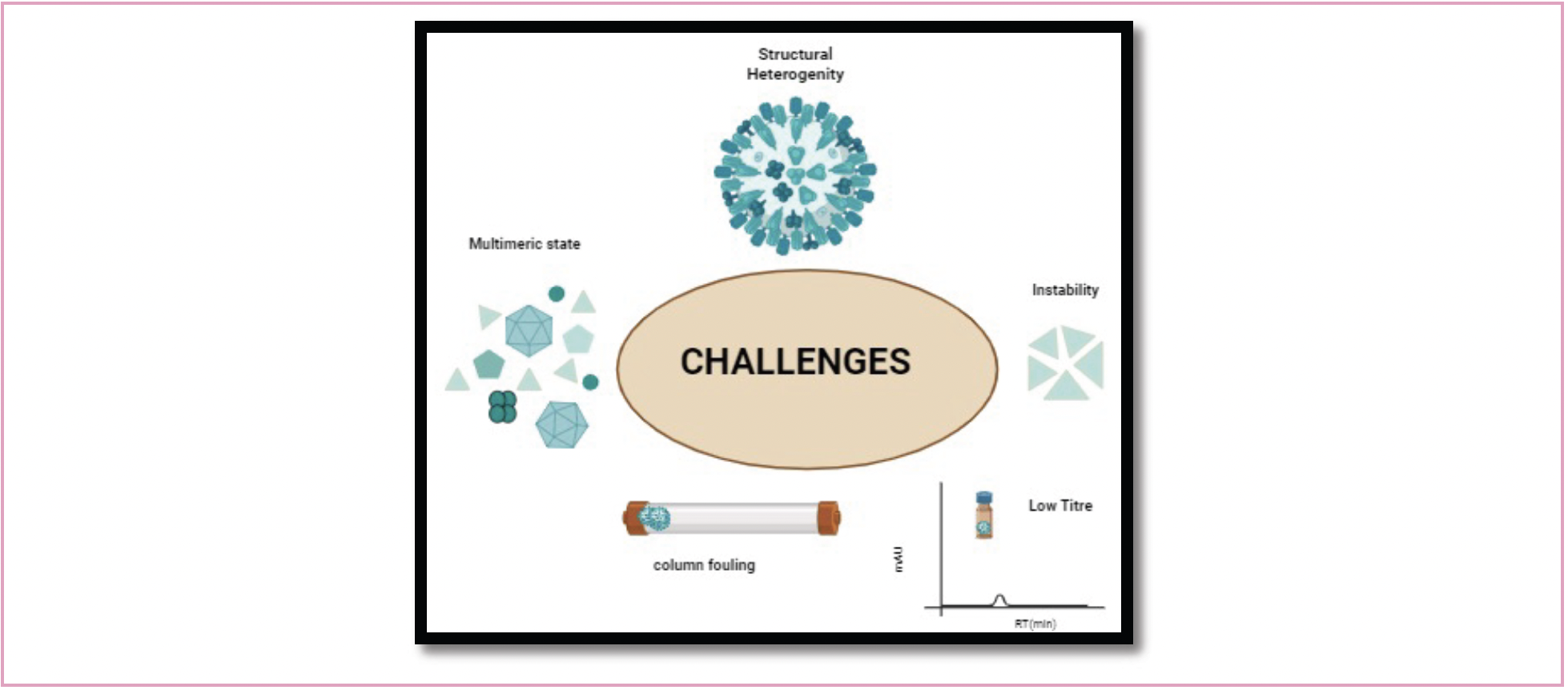
Artifacts are introduced by microscopic methods such as TEM and AFM because they require drying for sample preparation. AFM offers superior resolution compared to TEM. Nevertheless, artifacts can be minimized when VLPs are scanned in their natural state utilizing cryo-EM. However, in general, microscopic methods are non-quantitative, and require laborious sample preparations. Biophysical techniques like DLS only work with monodisperse samples; however, DLS suffers from a major flaw in that the signal is disproportionately impacted by larger particles. Therefore, if there is a broad particle distribution, accuracy of measuring smaller particles is significantly compromised. Other biophysical techniques like SEC-MALS, AF4-MALS and electrospray-differential mobility analysis (ES-DMA) offer size separation before size measurement, which is more accurate and robust, but these techniques too are semi-quantitative. SDS-PAGE and western blotting are time-consuming and tiresome. Quantification through bicinchoninic acid assay, Bradford assay, and Lowry assays is sensitive to the use of detergents like urea, and also give total protein quantification rather than VLP structural proteins (6,7) (Figure 2).
FIGURE 2: Challenges in analytical characterization of VLP through HPLC-based techniques.
Why HPLC-Based Methods?
Chromatography has been the most widely used technique for analytical characterization and quality control monitoring of biotherapeutics for decades. Reasons for this include the simplicity of operation, high resolution, robustness, sensitivity, and continued innovation in development of chromatography media that facilitates protein separation based on a variety of physicochemical characteristics, such as charge, hydrophobicity, and size (8). Beginning in the early 1980s, attempts were made to isolate influenza viral proteins using a variety of chromatographic techniques, including size-exclusion chromatography (SEC), reversed-phase chromatography (RP-HPLC), and ion exchange chromatography (IEX). However, significant challenges are faced when performing chromatographic characterization of VLPs.
Challenges Faced During HPLC-Based Characterization of VLPs
Multimeric State Through SEC
In comparison to other biotherapeutic proteins, VLPs are very large in size. Furthermore, most of the HPLC columns are designed for analysis of therapeutic proteins (such as monoclonal antibodies). As a result, when used for VLP characterization, the VLPs simply are eluted into void volumes of these columns. Very few bigger pore-size columns are available commercially for identification and quantification of intact VLPs. VLPs can be found in several molecular states in in-process samples. For instance, human papillomavirus (HPV) VLP is known to exist in three different states: monomeric (55 KD), pentameric (capsomeres), and completely assembled (19 megadaltons). It is impossible to identify and accurately measure each of these three states in a single HPLC analysis. Recent development of two-dimensional liquid chromatography (2D-LC) has made it possible to use multiple pore-size SEC columns to better characterize the different molecular states. Researchers have coupled a 2D-LC system with refractive index (RI) and a MALS detector and effectively separated, disassembled—intact—and aggregated VLP species in a single run using SEC columns in both dimensions (9). In the first dimension, a 250 Å SEC column was used and the peaks eluted in void volume were transferred to the second dimension through a heart-cut method. The second dimension SEC column has a pore size of 2000 Å, an ideal column used for intact VLP separation (9).
Molecular Heterogeneity Through RP–HPLC
VLPs are also structurally heterogeneous, as they are composed of many structural proteins, and, in certain instances, structural proteins of the virus combine with the proteins of the host cell to generate irregularly shaped VLPs. Therefore, using SEC alone to characterize VLPs may be incomplete. A better approach in such a case would be to use chromatographic techniques that separate based on other physiochemical properties like hydrophobicity. However, VLPs must be reduced from a fully assembled state to a monomeric state to perform reversed-phase chromatography. It is possible to convert completely formed VLP into a monomeric state by efficient sample preparations with the optimum amount of denaturants and reducing agents. Selecting the appropriate carbon chain column and pore size together yields improved resolution and accurate quantification than SDS-PAGE. The molecular weight of the desired species can be confirmed using reversed-phase chromatography by coupling LC with mass spectrometry. By reducing VLPs and digesting the resultant peptides with the trypsin enzyme, reversed phase chromatography in combination with tandem mass spectrometry (MS/MS) provides sequence coverage of the molecule with the database sequence (known as peptide mapping). In a recent publication, we have demonstrated efficient sample preparation for any process stage for characterization of HPV VLP (10). Crude samples were precipitated with 30% ammonium sulfate solution and the pellet was dissolved in 8M guanidine hydrochloride (GuHCl) and 100 mM dl-dithiothreitol (DTT) to effectively reduce the 19 megadalton molecule into its monomeric forms of 55 kilodaltons. The reversed-phase chromatography method was validated to identify HPV VLP L1 proteins in 20 min.
Column Fouling
In many cases, nucleic acids act as scaffolds for assembly of viral structural proteins. The use of optimal mobile phases that preserve the native state of VLPs and reduce the interactions of nucleic acids with columns help in achieving better resolution. Often, SEC mobile phases constitute higher NaCl concentrations than usual, along with other components like ethylenediaminetetraacetic acid (EDTA) to minimize sample and resin interactions. In reversed-phase chromatography, enveloped viruses exert column interactions due to their outer lipid membrane, and zwitterionic detergents are used to solubilize surface envelop glycoproteins and to also minimise the column interactions. In the case of chikungunya virus VLP, researchers have incubated samples with Zwittergent 3–12 detergent before analysis (7). The same strategy can be followed to release hemagglutinin (HA) proteins of influenza VLP. Other researchers have incubated influenza whole virus (inactivated vaccine) with 1% zwittergent 3–14 for 10 minutes at room temperature before analysis through reversed-phase chromatography (11).
Detection
VLP samples typically have lower titres than monoclonal antibody samples. When detecting at 280 nm UV wavelength, it is advantageous to inject highly concentrated samples or to use UV wavelengths of 220 nm in addition to 280 nm. Using more sensitive detectors, like fluorescence detectors, would be the best choice.
Stability
Because of significant instability that is inherent in VLPs, they frequently fragment or agglomerate to create irregularly shaped particles. VLPs of asymmetrical shapes are not very potent. Therefore, maintaining ideal pH conditions and using optimum amounts of EDTA can preserve the VLP’s native state and size. DTT and EDTA have been used to reduce the SV40 VLP's ability to withstand mechanical stress, leading to populations of varying sizes (12). Any negatively charged polymer, like DNA, promotes interpentameric (capsomers) interactions that lead to homogeneous populations.
HPLC in Conjunction with MS
HPLC in conjunction with MS provides detailed information on VLP components, primary structure, glycosylation patterns, post-translational modifications (PTMs), and also chemical modifications. With the advent of several sensitive MS techniques like orbital trap MS and charge detection–mass spectrometry (CD–MS), even knowing the molecular weight of fully assembled VLPs has been made possible.
Electrospray Ionization (ESI)-MS
VLPs are highly heterogeneous, and very large in size. This limits the application of MS in analyzing intact (fully assembled) VLPs. Electrospray ionization (ESI) is the most commonly used ion-generating technique for proteins. It can be coupled to liquid chromatography (LC) or capillary electrophoresis (CE) for protein separation. ESI can generate ions directly from solution, and thereby produce multiply charged ions, enabling determination of intact mass up to 1 mega Dalton. In some cases, there can be signal overlap and deviations in observed ionic mass from actual analyte mass, due to multiple charged ions and adduct formation. This complicates the interpretation of mass spectra, especially in heterogeneous ions, because of poorly resolved signal distribution.
Matrix-Assisted Laser Desorption Ionization (MALDI)-MS
MALDI-MS produces a singly charged state. Sometimes, ions may be multiply charged, especially in the case of larger molecules, and the low extent of multiple charging requires the use of the time-of-flight mass analyzers. Also, the adduct formation leads to deviation in ionic mass from the neutral analyte. MALDI-MS is not compatible with either LC or CE. Hence, it is poorly suited to native MS and requires chemical linkers for analyzing non-covalent interactions.
Ion Mobility–Mass Spectrometry (IM-MS)
IM-MS plays an efficient role in characterization of heterogeneous molecules up to a few kilodaltons. It resolves gas phase ions based on differences in their collision cross-section. However, it is incompatible with large heterogenous molecules (13).
Charge Detection Mass Spectrometry (CD–MS)
CD–MS plays a prominent role in determining the mass of large heterogenous molecules where conventional techniques fail because of overlapping signals from complex ion distributions. CD– MS is a single ion approach that measures mass by estimating both mass-to-charge ratio (m/z) and charge of the ion. This gives an accurate mass of molecules over a broad size range. CD–MS resolves overlapping signals by transferring ions to a lower charge state. In CD–MS, ionization occurs through ESI or MALDI and the ion passes through a conducting tube, m/z is measured by the passage time or oscillation frequency, and z is determined from the magnitude of the charge. This technique is highly sensitive, as it measures the m/z and charge of a single particle and therefore requires very little sample. Researchers have used CD–MS to analyze the mass of a live attenuated virus (RotaTeq), a VLP (Gardasil 9), and a complete viral vaccination as an inactivated polio vaccine (IPOL) (14). In this study, ions were generated through nano-ESI and multiple phases of differential pumping were employed to thermalize and concentrate positively charged ions, accelerating them to an energy of 100 eV/z. An electrostatic linear ion trap (ELIT), which traps ions for a duration of 100 milliseconds, received a narrow energy distribution from a dual hemispherical deflection energy analyzer. A charge-sensitive amplifier picked up the oscillating ions' signal, which were then converted to digital form and examined using quick Fourier transforms. The researchers reported that CD–MS is a reliable, sensitive method that can quickly ascertain the mass of large, heterogeneous molecules (14).
Conclusion
VLPs are large heterogenous molecules and their characterization poses significant challenges, due to their large size, heterogeneity, low titres, and instability of intermediate molecular states. Traditional methods of analytical characterization include a wide variety of microscopic, biochemical and biophysical techniques. However, although all of these techniques do offer useful information about morphology or structural composition, they are semi-quantitative, tedious, and time-consuming. In contrast, HPLC offers the possibility of a robust, simple, and sensitive analysis. Most commonly used chromatographic techniques include size-exclusion chromatography for assessing morphology and molecular state (fully assembled, capsomere, or monomeric state) and reversed-phase chromatography for purity and structural composition. However, there are significant challenges that are faced when performing HPLC-based characterization of VLPs. These exist because of the large size (and, at times, improper assembly) of viral structural proteins. Furthermore, column fouling (because of strong binding or the instability of the VLP) can also be a hurdle. In this article, we reviewed the challenges—as well as solutions—that researchers have proposed. Intact VLP characterization through ESI-MS is not possible because of multiply charged states that would lead to overlapping signal and deviation because of adduct formation. CD–MS is the ideal choice, as it is a single ion approach and gives both charge and m/z ratio.
References
(1) Mohsen, M. O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M. F. Major Findings and Recent Advances in Virus–Like Particle (VLP)-Based Vaccines. Semin. Immunol. 2017, 34, 123–132. DOI: 10.1016/j.smim.2017.08.014
(2) Lua, L. H. L.; Connors, N. K.; Sainsbury, F.; Chuan, Y. P.; Wibowo, N; Middelberg, A. P. J. Bioengineering Virus-Like Particles as Vaccines. Biotechnol. Bioeng. 2014, 111, 425–440. DOI: 10.1002/bit.25159
(3) Mohsen, M. O.; Bachmann, M. F. Virus-Like Particle Vaccinology, from Bench to Bedside. Cell Mol. Immunol. 2022, 19, 993–1011. DOI: 10.1038/s41423-022-00897-8
(4) Steppert, P.; Burgstaller, D.; Klausberger, M.; Tover, A.; Berger, E.; Jungbauer, A.; Quantification and Characterization of Virus-Like Particles by Size-Exclusion Chromatography and Nanoparticle Tracking Analysis. J. Chromatogr. A 2017, 487, 89–99. DOI: 10.1016/j.chroma.2016.12.085
(5) Huang, X.; Wang, X.; Zhang, J.; Xia, N.; Zhao, Q. Escherichia coli-Derived Virus-Like Particles in Vaccine Development. npj Vaccines 2017, 2. DOI: 10.1038/s41541-017-0006-8
(6) Gupta, R.; Arora, K.; Roy, S. S.; Joseph, A.; Rastogi, R.; Arora, N. M.; Kundu, P. K. Platforms, Advances, and Technical Challenges in Virus-Like Particles-Based Vaccines. Front. Immunol. 2023, 14. DOI: 10.3389/fimmu.2023.1123805
(7) Shytuhina, A.; Pristatsky, P.; He, J.; Casimiro, D. R.; Schwartz, R. M.; Hoang, V. M.; Ha, S. Development and Application of a Reversed-Phase High-Performance Liquid Chromatographic Method for Quantitation and Characterization of a Chikungunya Virus-Like Particle Vaccine. J. Chromatogr. A 2014, 1364, 192–197, DOI: 10.1016/j.chroma.2014.05.087
(8) Sharma, V. K.; Sharma, I.; Glick, J. The Expanding Role of Mass Spectrometry in the Field of Vaccine Development. Mass Spectrom. Rev. 2020, 39 (1–2), 83–104. DOI: 10.1002/mas.21571
(9) Barrientos, R. C.; Singh, A. N.; Ukaegbu, O.; et al. Two-Dimensional SEC-SEC-UV-MALS-dRI Workflow for Streamlined Analysis and Characterization of Biopharmaceuticals. Anal. Chem. 2024, 96 (12), 4960–4968. DOI: 10.1021/acs.analchem.3c05969
(10) Peruri, V.; Bhattacharya, S.; Rathore, A. S. Reversed Phase High Performance Liquid Chromatography for Monitoring Type-16 Human Papilloma Virus Like Particles. J. of Chromatogr. Open 2024, 5, 100107. DOI: 10.1016/j.jcoa.2023.100107
(11) Kapteyn, J. C.; Porre, A. M.; de Rond, E. J. P.; et al. HPLC-Based Quantification of Haemagglutinin in the Production of Egg- and MDCK Cell-Derived Influenza Virus Seasonal and Pandemic Vaccines. Vaccine 2009, 27, 1468–1477. DOI: 10.1016/j.vaccine.2008.11.113
(12) van Rosmalen, M. G. M.; Li, C.; Zlotnick, A.; Wuite, G. J. L.; Roos, W. H. Effect of dsDNA on the Assembly Pathway and Mechanical Strength of SV40 VP1 Virus-Like Particles. Biophys. J. 2018, 115 (9), 1656–1665. DOI: 10.1016/j.bpj.2018.07.044
(13) Hickey, J. M.; Sahni, N.; Toth IV, R. T.; Kumru, O. S.; Joshi, S. B.; Middaugh, C. R.; Volkin, D. B. Challenges and Opportunities of Using Liquid Chromatography and Mass Spectrometry Methods to Develop Complex Vaccine Antigens as Pharmaceutical Dosage Forms. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1032, 23–38. DOI: 10.1016/j.jchromb.2016.04.001
(14) Miller, L. M.; Bond, K. M.; Draper, B. E.; Jarrold, M. F. Characterization of Classical Vaccines by Charge Detection Mass Spectrometry. Anal. Chem. 2021, 93 (35), 11965–11972. DOI: 10.1021/acs.analchem.1c01893
ABOUT THE EDITORS
Anurag S. Rathore is a professor in the Department of Chemical Engineering at the Indian Institute of Technology in Delhi, India.

Vineela Peruri is a PhD student in the analytical division at the Centre of Excellence for Biopharmaceutical Technology under the guidance of Professor Anurag S. Rathore, Department of Chemical Engineering, Indian Institute of Technology, Delhi.

Jared R. Auclair is Interim Dean at the College of Professional Studies, Vice Provost Research Economic Development and Director of Bioinnovation at Northeastern University, in Boston, Massachusetts. He is also the Director of Biotechnology and Informatics, as well as the Director of the Biopharmaceutical Analysis Training Laboratory.


Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.
Troubleshooting Everywhere! An Assortment of Topics from Pittcon 2025
April 5th 2025In this installment of “LC Troubleshooting,” Dwight Stoll touches on highlights from Pittcon 2025 talks, as well as troubleshooting advice distilled from a lifetime of work in separation science by LCGC Award winner Christopher Pohl.














